The nature of nurture: Genetic influence on “environmental” measures
Robert Plomin and C. S. Bergeman.
Behavioral and Brain Sciences (1991) 14, 373-427.
Abstracts. Evidence for genetic influence on environmental measures will emerge in quantitative genetic analyses if genetically influenced characteristics of individuals are assessed by these environmental measures. Recent twin and adoption studies indicate substantial genetic influence when measures of the environment are treated as phenotypes in genetic analyses. Genetic influence has been documented for measures as diverse as videotaped observations of parental behavior toward their children, ratings by parents and children of their family environment, and ratings of peer groups, social support, and life events. Evidence for genetic influence on environmental measures includes some of the most widely used measures of environment – the Home Observation for Measurement of the Environment, the Family Environment Scales, and the Social Readjustment Rating Scale of life events, for example. The goal of this article is to document and discuss these findings and to elicit commentary that might help to shape the course of research on this topic, which has far-reaching implications for the behavioral and brain sciences.
1. Introduction
In the traditional stimulus-response model, the environment is independent of the organism. It is something imposed on the organism from the outside, like an electrical shock applied to the paw of a mouse. This view allows no role for DNA, because the organism has nothing to do with the environment that impinges on it. In the social and behavioral sciences, however, measures of the environment often must blur the distinction between environment and organism in the search for experiences that affect the individual.
For example, in developmental psychology, the parents are viewed as a major source of environmental influence: Many measures of home environment directly assess parental behaviors – parental responsiveness, for example. Parental behavior assessed on measures of home environment might well reflect organismic characteristics, such as the personality of the parents (Plomin 1986), as well as that of the children (Bell 1968). Although other measures of the home environment — parental education and occupation, and the perennial item, the number of books in the home – assess parental behavior less obviously and are unlikely to be influenced by characteristics of children, these measures may nonetheless reflect such organismic characteristics of parents as their IQ. Environmental measures outside the home (e.g., peers, life events, and social support) might also involve characteristics of the organism, as individuals select, modify, and even create their environments.
Finally, the majority of environmental measures used in the social and behavioral sciences involve self-report; self-perceptions filter through the complex feelings, personality, and cognition of the individual and thus incorporate the organism in the measure of the environment. A few measures of the environment may have nothing to do with the organism (e.g., accidents and illnesses or classroom size) although organismic involvement or lack of involvement must be assessed rather than assumed. For example, such personality traits as risk-taking might influence accident proneness, individuals differ in their susceptibility to disease, and classroom size might be related to socioeconomic status.
Blurring the distinction between environment and organism may be necessary for identifying environmental influences relevant to behavioral development; indeed, this seems essential to study the environment as experienced. Including characteristics of the organism in measures of the environment has an important implication, however: The ubiquitous genetic influence found for most organismic characteristics can result in genetic influence on ostensible measures of the environment.
The basic quantitative genetic model is widely used to break down the variance of such phenotypes as behavior into genetic and environmental components of variance (e.g., Plomin, DeFries et al. 1990). Quantitative genetic analyses using twin and adoption designs have shown some hereditary influence on nearly all behavioral phenotypes investigated. Environmental measures are now being considered within the quantitative genetic model as a measured index of the anonymous environmental component of variance. (See Figure 1a.) For example, in behavioral genetic analyses of the variance of children’s IQ scores, specific measures of parenting can be incorporated into a parent-offspring model as an index of environmental variance affecting children’s IQ; one can thereby assess the contribution of the environmental index to children’s IQ independent of parental IQ (Bergeman & Plomin 1988; Coon et al. 1990; Plomin et al. 1985; Rice et al. 1988; Thompson et al. 1986). These analyses also indicate the extent to which the environmental index may be associated genetically with the IQ of parents and children.
This is an appropriate way to address genetic effects on an environmental measure in a quantitative genetic framework. This traditional approach, however, is limited to finding genetic influences on an environmental measure only to the extent that the environmental measure relates to the particular behavioral phenotype under investigation. Specifically, genetic influence on an environmental measure will be estimated in these analyses only if genotype-environment correlation occurs between the measure of the environment and the particular behavioral phenotype (Plomin 1986). Genotype-environment correlation refers literally to a correlation between genetic and environmental deviations as they affect a particular phenotype. For example, a genotype-environment correlation occurs for a particular measure of behavior (e.g., musical ability) if children are exposed to musical training on the basis of their genetic propensities. In addition to this quantitative genetic perspective on genotype-environment correlation, sociobiologists have begun to consider genotype-environment correlation at the level of comparisons between species (Jones 1980; Lumsden 1989).
A more radical position is that environmental measures should be considered as phenotypes themselves. (See Figure 1b.) That is, a measure of the environment can be treated as a phenotype and analyzed using quantitative genetic methods to break down its variance into genetic and environmental components. This environment-as-phenotype approach allows us to turn the spotlight on the environmental measures themselves, exploring the relative degree of genetic and environmental influence on these measures regardless of their association with a particular phenotype, unlike genotype-environment correlation analyses, which limit the search for genetic influence on an environmental measure to its association with a particular phenotype. It should be emphasized that our argument is that measures of the environment – not the environment itself – should be conceptualized as phenotypes. Environments have no DNA and can show no genetic influence. Measures of the environment, as our examples suggest, may be perfused with characteristics of individuals, however. To the extent that this is the case, measures of the environment can show genetic influence. Consider such an environmental construct as parental responsiveness. We might think of this as existing “out there,” independent of individuals. When we measure the construct, however, we are in fact measuring parental behavior, and this measure can be analyzed as a phenotype in quantitative genetic analyses to determine the extent to which interindividual genetic and environmental differences contribute to phenotypic variance for this measure. If the measure is really “out there,” independent of individuals, it will show no genetic influence.
Although it sounds odd to consider environmental influence on a measure of the environment, we again emphasize the word measure and consider the example of parental responsiveness. If a measure of parental responsiveness shows no genetic influence in quantitative genetic analyses, this means that differences in responsiveness among parents is the result of nongenetic factors. In quantitative genetics, environmental influence refers to this residual component of variance, which is much broader than the systematic psychosocial environments that behavioral scientists usually consider. For example, it includes such nonhereditary biological factors as illness and accidents, nutrition, and even nonhereditary events related to DNA itself.
This way, the environment-as-phenotype perspective goes beyond genotype-environment correlation in considering genetic influence on measures of the environment. For example, in terms of genotype-environment correlation, a measure of parental responsiveness would be viewed as an index of the environmental component of variance for a behavioral measure such as IQ (see Figure 1a). Genetic influence on this environmental index would be estimated in the context of a parent-offspring model of some specific phenotype (e.g., IQ), and genetic influence would be found only to the extent that the environmental index is associated with the IQ of the parent and that of the offspring. In contrast, the environment-as-phenotype approach might well find evidence for genetic influence on parental responsiveness (in an observational study of twin parents’ responsiveness towards their children, for example) even if parental responsiveness is not at all related to IQ. That is, genetic influence on parental responsiveness might come from other sources – personality, for example – or, as discussed later, from genetically influenced patterns of interaction with one’s environment that are independent of our traditional dimensions of personality. Although this distinction between environment-as-phenotype and genotype-environment correlation is important for finding evidence for genetic influence on environmental measures, it should be noted that in terms of developmental processes (in contrast to components of variance) genetic influence on any phenotype is best conceptualized in terms of genotype-environment correlation in which genetic dispositions work themselves out in transactions with the environment (Scarr & McCartney 1983).
One might argue that finding genetic influence on environmental measures means that the measures are not really measures of the environment. If one adopts this position, then by definition there can be no genetic influence on environmental measures. Although we disagree with this position, the present article can be reformulated to accommodate it: Our goal is to explore the extent of genetic influence on measures that are thought to be and are widely used as indices of the environment. Regardless of whether one would attempt to define away genetic influence on measures of the environment, it should be of interest to learn the extent to which genetic factors affect available measures of the environment.
The goal of this article is to document the importance of genetic influence on measures of the environment. For this reason, the body of the article consists of a litany of studies using diverse methods and measures with data relevant to the issue of genetic influence on environmental measures. Recognizing that environmental measures can be significantly influenced by heredity leads to questions about mechanisms that might mediate this genetic influence. In the last section (sect. 3) we allow ourselves to speculate about mechanisms that might underlie genetic influence on environmental measures. In the absence of empirical evidence on this topic, however, we keep these speculations brief. We hope that success in achieving our goal of providing convincing evidence of genetic influence, on environmental measures will stimulate the next steps in this program of research on the nature of nurture, steps that include investigating the antecedents and sequelae of genetic influence on environmental measures.
2. Evidence for genetic influence on measures of the environment
Most of the relevant research on the nature of nurture involves proximal measures of the family environment, as distinguished from such distal measures as SES (socioeconomic status) and parental education. After a lengthy review of work on such proximal measures of family environment, the few available genetic analyses of other environmental measures are described – for example, SES and education, television viewing, peers, social support, and life events.
Space does not allow us to present the basic concepts and methods of quantitative genetics. Background information about twin and adoption methods and analyses is available in an earlier Behavioral and Brain Sciences article (Plomin & Daniels 1987) and in a recent textbook (Plomin, DeFries et al. 1990).
2.1. Proximal family environment
At least three measurement issues can affect the genetic contribution to proximal measures of family environment: method, source, and target. Methodologically, measures of the family environment vary from completely objective ones – videotape observations of mothers interacting with their children, for example – to subjective ratings by parents and by children. Such an objective measure of childrearing as videotaped interactions between parent and child could incorporate genetically influenced characteristics of the parent as well as those of the child. As mentioned earlier, ratings can contribute additional genetic influence. For ratings, the magnitude and type of genetic influence could differ as a function of the source of information – typically, the parent, the child, or an observer in the home.
Method and source potentially affect the results of any measures, not just genetic estimates of family environment. A third issue is more specific to genetic analyses of measures of family environment: the target of the genetic analysis. For example, in comparisons between identical and fraternal twins, the twins could be the parents or the children. In an observational study of twin parents in which the environmental measure is the childrearing of these parents in their separate families, any characteristics of the parents might be relevant to their childrearing. In contrast, in an observational study of twin children in which the childrearing of the parents of the children is assessed, genetic influence is limited to characteristics of children to which parents respond. That is, genetically influenced characteristics of the parents will not contribute genetic variance to measures of childrearing in the typical case in which the twins are the children, except for the diluted hereditary resemblance between adult parents’ characteristics and related characteristics of their children.
Thus, for observational studies, it could be hypothesized that childrearing measures show greater genetic influence in studies in which the target twins are parents rather than children, but unfortunately, no such studies have been reported. When the method involves ratings rather than observations, genetic influence could be adduced as perceptions filter through genetically influenced characteristics such as personality (regardless of whether the target twins are parents or children). There may be important interactions between method, source, and target as well. For example, in the case in which ratings are made by parents and the target twins are the parents’ children,’ genetic influence on the subjective processes entailed in the parents’ ratings will not show up in analyses of their twin children. In contrast, when ratings are made by the twin children themselves, genetic influence on the children’s subjective processes related to their ratings will be incorporated in this assessment of the family environment.
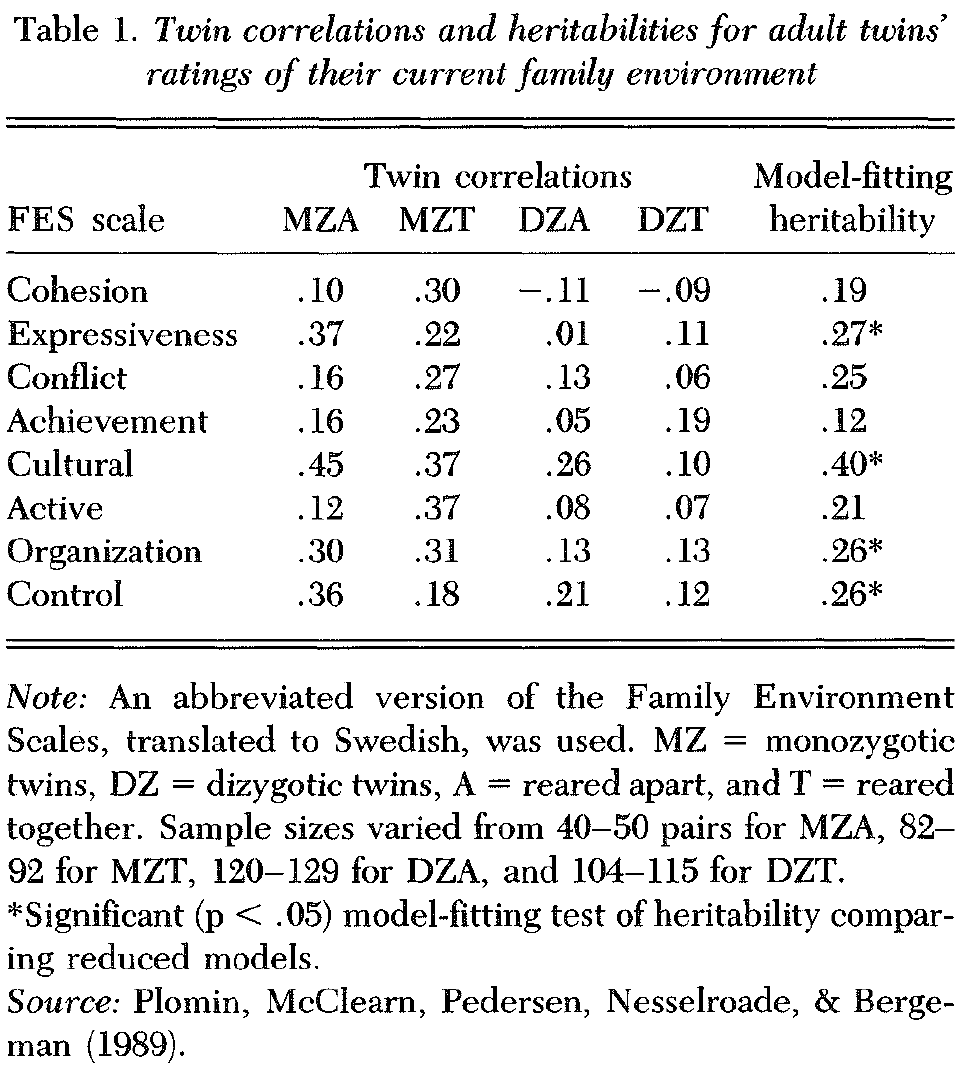
2.1.1. Studies of parents as targets. Only one genetic investigation of the family environment studied parents rather than children as targets, and this was a questionnaire study (Plomin, McClearn et al. 1989). Moreover, parenting per se was not assessed, but rather perceptions of the general family environment, with the widely used Family Environment Scales (FES; Moos & Moos 1981). To the extent that the FES assesses not only characteristics of the parents but also characteristics of the spouse and children, it could dilute estimates of genetic influence when parents are the target of the genetic analysis. The study is part of the Swedish Adoption Twin Study of Aging (SATSA), mentioned several times in this article, which uses one of the most powerful designs in the armamentarium of behavioral genetics: identical (MZ) and fraternal (DZ) twins reared apart (MZA and DZA) and matched groups of MZ and DZ twins reared together (MZT and DZT; McClearn et al, submitted; Pedersen et al., in press). An abbreviated version of the FES was completed by 179 reared-apart twin pairs and 207 reared-together pairs in relation to the twins’ current family, that is, the family consisting of the adult subject, spouse, and children. The results, summarized in Table 1, suggest that genetic factors affect the FES. The average MZA correlation is .25, ranging from .10 for cohesion to .45 for culture. Because the MZA correlation directly estimates heritability, this suggests that about 25% of the variance of the FES scales is the result of genetic influence. The average DZ correlation is .10, suggesting a heritability of about 20%. The average MZT correlation is .28 and the average DZT correlation is .09; thus, the classical twin estimate of heritability that doubles the difference between the MZT and DZT correlations is 38%. The estimate of heritability that doubles the difference between the MZA and DZA correlations is 30%. The average of these four estimates suggests that the heritability of the FES scales is about 25%, with the lowest heritability for achievement (14%) and the highest for culture (47%). Because of the large standard errors of correlations, considerable sampling fluctuation can be seen. Model-fitting analyses are particularly helpful in this regard because they analyze data from all four groups simultaneously and provide tests of statistical significance for parameter estimates. The SATSA model used in these and subsequent SATSA reports is presented in detail elsewhere (Plomin, McClearn et al. 1988; Plomin, Pedersen et al. 1988). The results of model fitting verify the conclusions reached on the basis of examining the simple correlations: The average model-fitting heritability estimate is 24%, with the lowest estimate for achievement (12%) and the highest estimate for culture (40%). Heritability estimates were statistically significant (p < .05) for Expressiveness, Cultural, Organization, and Control, and marginally significant (p < . 10) for Conflict and Active.
Thus, this first study of FES ratings in which the parents are the target twins suggests that about a quarter of the variance is the result of genetic differences among individuals, which is similar to the magnitude of genetic influence on personality measures in the same study (McClearn et al., submitted). The most surprising of these results is that the reared-apart twins rated different families similarly, and that this was the case to a greater extent for MZA than for DZA twins. Unless the rearing families of separated twins were similar (because of selective placement), this evidence for genetic influence allows two interpretations: Either heredity is involved in general perceptions (e.g., “looking at life through rose-colored glasses”) or members of the two families in fact responded similarly to genetically influenced characteristics of the separated twins.
2.1.2. Studies of children: Twin studies. In all other genetic studies of the family environment, the targets are children rather than parents; most of these studies also use parental and child ratings rather than observations. The earliest relevant work was not conducted for the purpose of investigating genetic influence on measures of the family environment. The goal of the research was to address the “equal environments” assumption of the twin method by investigating whether MZ twins are treated more similarly than DZ twins (Lehtovaara 1938; Loehlin & Nichols 1976; Smith 1965; Wilson 1934; Zazzo 1960). Although MZ twins were found to be treated more similarly by their parents for some measures, the bottom line was that such MZ-DZ differences in treatment do not relate to twin differences in behavior (Plomin, DeFries & McClearn 1990).
It was not asked, however, why ratings of MZ twins’ treatment are more similar than those of DZ twins in the first place; genetic influence on these measures of family environment could be part of the answer. An alternative possibility is that attributional biases because of labelling twin pairs as MZ or DZ could lead to these results. This is unlikely, however, because research with twins whose zygosity is mistaken by their parents has shown that true rather than mistaken zygosity governs twin similarity (Scarr 1968; Scarr & Carter-Saltzman 1979).
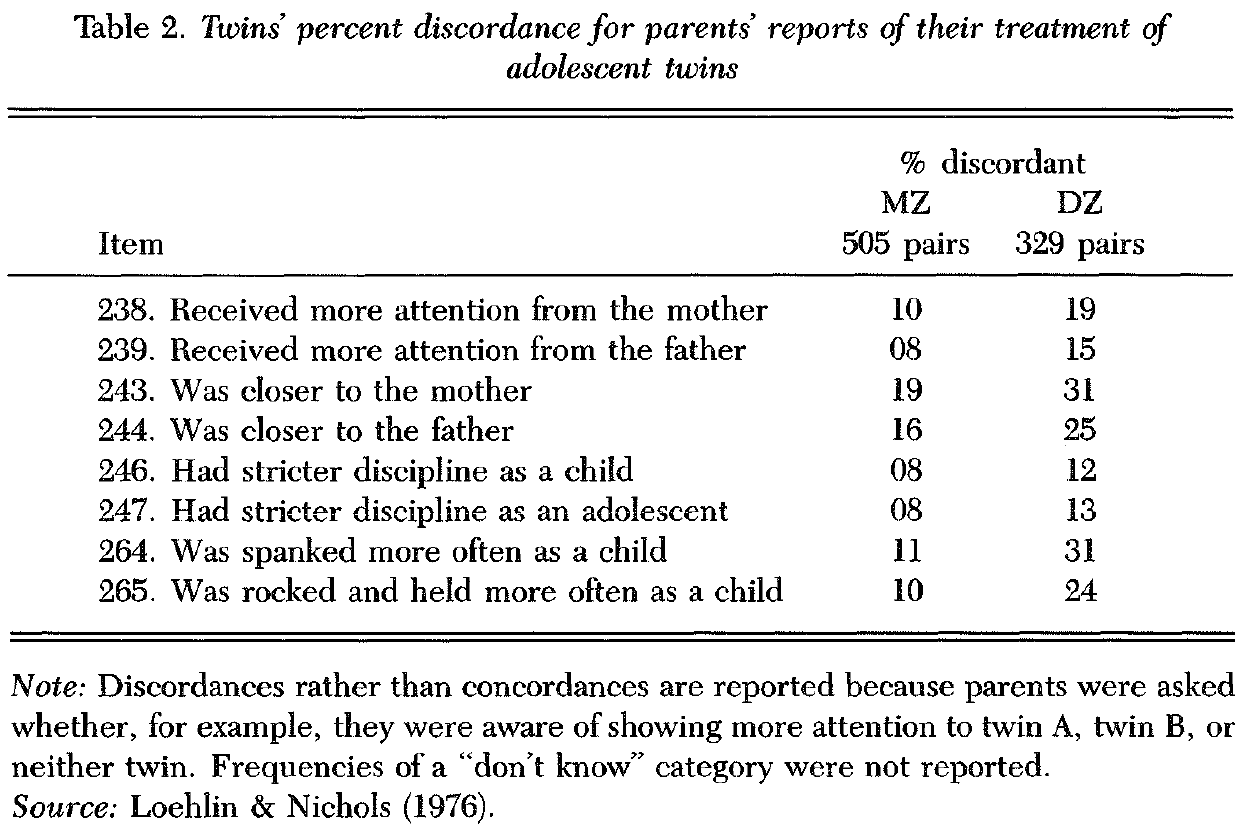
2.1.2.1. Loehlin & Nichols (1976) study. The largest and most thorough study of this type involved 850 pairs of high school twins (Loehlin & Nichols 1976). Among the more than 1,000 items in the study (with twin results for each item helpfully included in an appendix) are several items that involve parents’ ratings and children’s ratings of childrearing variables. The parental rating items consist of same-different judgments concerning the parents’ treatment of each child. For most items, parents rarely indicated that they treated their children differently, regardless of whether the twin children were identical or fraternal. Similarly, parent ratings in another study of twins from 1 to 6 years of age yielded twin correlations in excess of .90 for measures of childrearing (Cohen et al. 1977). Taken at face value, these results suggest that parents do not respond to genetic differences between their children, although the possibility looms large that parents deny differential treatment of their children because this goes against social conventions. Observational studies might tell a different story.
For a few items in the Loehlin & Nichols study, however, parents reported that they treated their children differently to some extent, which makes it possible to ask whether differential treatment is greater for DZ than for MZ pairs. Table 2 lists the percentage of discordant MZ and DZ pairs for these items. Discordances are consistently greater for DZ twins than for MZ twins, which suggests some genetic influence. The average percent discordance is 11% for MZ twins and 21% for DZ twins. Although data of this type permit no precise estimate of the magnitude or significance of genetic influence, at least some modest genetic influence is suggested by these results.
The Loehlin & Nichols data set also includes several items relating to the twin children’s perceptions of their parents’ treatment. Intraclass correlations for these quantitative ratings are listed in Table 3. Each item shows greater resemblance for MZ twins than for DZ twins – significantly so for all but one item – suggesting that heredity affects adolescents’ perceptions of their parents’ treatment. The average twin correlations are .55 and .34, respectively, for MZ and DZ twins for the items in Table 3, suggesting substantial genetic influence.
2.1.2.2. Lytton’s study. Another study motivated by the equal environments assumption of the twin method is important for several reasons, despite its small sample size (17 MZ, 29 DZ pairs; Lytton 1977; 1980). Although interview data were involved in the measures of parental treatment, the primary data were derived from observational ratings of mothers interacting with their twins. These observations of mother-child interaction appear to show greater differential behavior than do parental reports of differential treatment discussed in the previous section. Similar to the parental report items that show differential treatment, these observational data suggest that parents treated MZ twins more similarly than DZ twins: Seven parental treatment variables showed significantly greater variance within DZ pairs than within MZ pairs (twin correlations were not reported). These parental treatment variables included use of material rewards, amount of play, support of dependence, encouraging mature action, monitoring, use of reasoning, and play frequency.
The most interesting feature of this study was its coding of parent-initiated actions, defined as parental actions that were not preceded by a child’s action within the previous 10 seconds. These measures of parent-initiated action were summarized in four categories: command/prohibition, suggestion, positive action, and negative action. The only category of parent-initiated treatment that suggested genetic influence was parents’ suggestions. The author concluded that parents of MZ twins are more likely than parents of DZ twins to respond to rather than create greater similarity in their children, thus supporting the equal environments assumption of the twin method. In the present context, these results can be reinterpreted as indicating genetic influence on parental treatment in response to characteristics of children. This finding fits well with expectations of genetic influence on childrearing as discussed earlier: Because this is a study of twin children that involves objective observations of childrearing, we would expect the results to show genetic influence on parents’ play and monitoring that is in response to their children rather than on parent-initiated measures. This pioneering study is an exemplar of the type of research that is needed to understand the processes underlying genetic influences on environmental measures.
2.1.2.3. Rowe’s studies. The previous studies inadvertently obtained data relevant to genetic influence on measures of the environment in their investigations of the equal-environments assumption of the twin method. Two twin studies by David Rowe (1981; 1983) were the first with the explicit goal to assess genetic influence on environmental measures. Adolescent twins were asked to rate parental treatment in two separate studies using different environmental measures and different samples. In his first study, Rowe (1981) assessed three dimensions: acceptance-rejection, control-autonomy, and firm-lax control, using an abbreviated version of Schaefer’s Children’s Reports of Parental Behavior Inventory (Schaefer 1965). Results of these two twin studies are summarized in Table 4. For ratings of both mother and father, the twin results suggested a significant and substantial genetic influence on acceptance-rejection. The two control-related dimensions, however, showed no indication of genetic influence.
Similar results were obtained in a second study of adolescents’ ratings on the Family Environment Scales and included nontwin siblings in addition to twins. Two second-order factors were derived that are similar to the warmth and control dimensions typically found in child-rearing research. The warmth dimension (called acceptance-rejection) refers not only to affection but also to parents’ supportiveness toward the child. Control (restrictiveness-permissiveness) involves the parents’ attempts to set and enforce rules and to organize the child’s life. The twin correlations indicate significant genetic influence for the warmth dimension, but no genetic influence was found for the control dimension. It is noteworthy that nontwin siblings in this study were as similar as DZ twins for both the warmth and control dimensions, suggesting that twins are not more sensitive than nontwin siblings in terms of perceived differences in parental treatment toward them and their same-aged cotwins.
It is especially interesting that in both of Rowe’s studies parental warmth – but not parental control – showed genetic influence. Loehlin & Nichols’s data also provide some support for this hypothesis. In Table 2, the item, “Was spanked more often as a child,” is problematic for this hypothesis, because it yielded the greatest difference between MZ and DZ twin discordances. Although spanking seems to be a control item, it is different from the typical control item that assesses family organization (e.g., assignment of chores), and it could be argued that this item actually involves warmth more than control. This could be determined empirically if correlations were available between spanking items and other control items. If spanking is more a matter of warmth than control, this item’s apparent genetic influence would support the hypothesis of greater genetic influence for parental warmth than control. The other items in Table 2 tend to support the hypothesis. Other than the spanking item, the two items with the largest difference between MZ and DZ twins are warmth items: item 265 (“Was rocked and held more often as a child”) and item 243 (“Was closer to the mother”). Two clear control items (“Had stricter discipline as a child”; “Had stricter discipline as an adolescent”) showed the smallest differential treatment of MZ and DZ twins. The twins’ own ratings in Loehlin & Nichols’s study (Table 3) are measures of parental warmth rather than of control, and all these items suggest genetic influence.
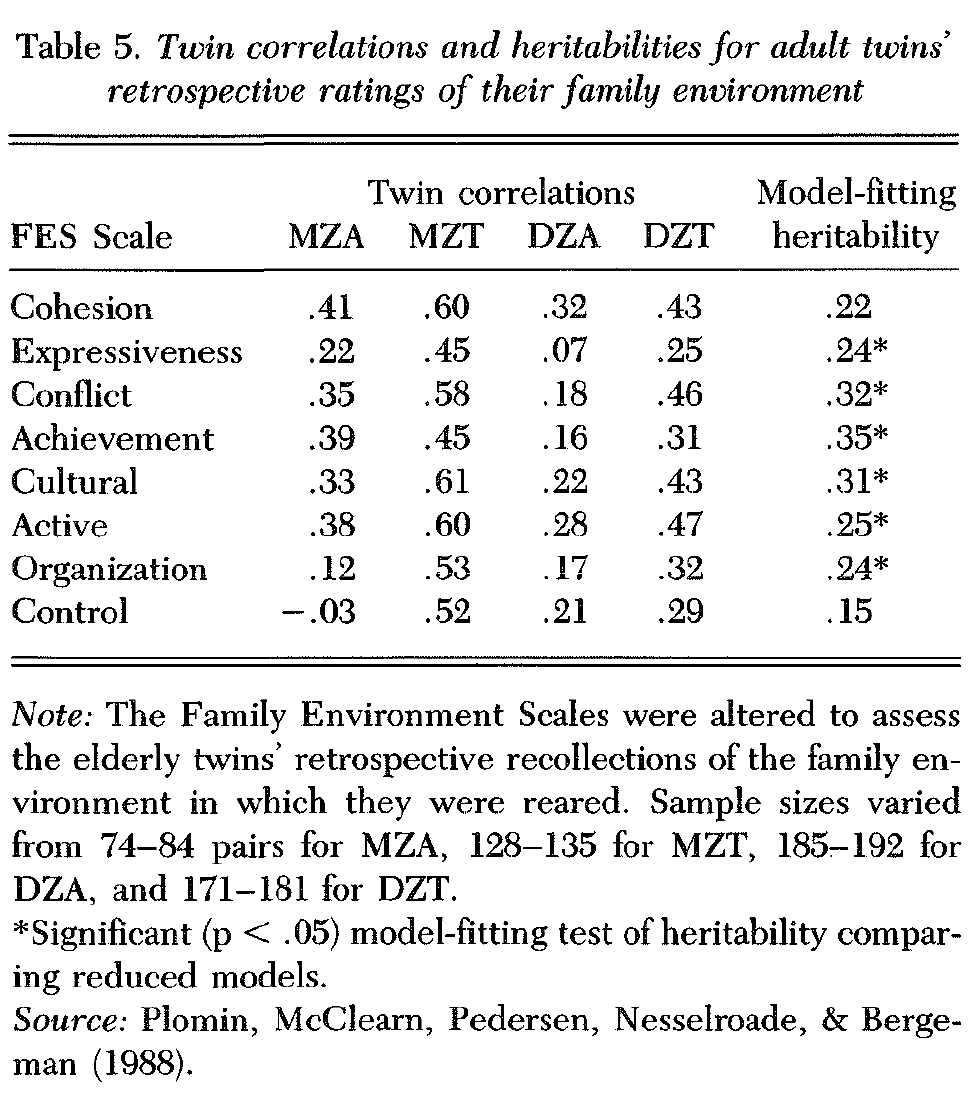
2.1.2.4. SATSA. The hypothesis that genetic influence is greater for parental warmth than for control was also supported in a SATSA analysis of retrospective ratings of childhood family environment viewed half a century later (Plomin, McClearn et al. 1988). Although the twins in this study are adults, it is a study of twins as children in the sense that they reported retrospectively about themselves as children in relation to the family environment in which they were reared.
Despite the procedural differences between SATSA and Rowe’s two studies of adolescents, the SATSA results, shown in Table 5, generally confirm Rowe’s findings. Perceptions of control show the lowest MZA correlation and the lowest model-fitting estimate of heritability, whereas warmth-related dimensions of expressiveness and conflict are significantly heritable; the cohesion scale shows only marginally significant (p < .06) heritability, however. Two second-order FES factors are similar to the factors reported by Rowe (1983). A warmth dimension called relationship consists of the cohesion, expressiveness, and conflict scales, and a control dimension called system maintenance includes the control and organization scales. Model-fitting heritability estimates for these warmth-related and control-related factors were .38 and .11, respectively, replicating Rowe’s finding of greater genetic influence for parental warmth than parental control.
SATSA adds to Rowe’s results by suggesting that genetic influence is found not only for warmth, but for nearly all FES dimensions other than control. The correlations for MZ twins reared apart from early in life are particularly impressive because these individuals were reared in different families. This could mean that genetic influence is in the eye of the beholder, that is, heredity may be involved in subjective characteristics that affect perceptions of the family environment. It is also possible, however, that members of the two families responded similarly to genetically influenced characteristics of the separated MZ twins.
These results may be especially pertinent to a new area of attachment research that focuses on adult parents’ descriptions of their relationships with their parents when they were children (Main et al. 1985). The possibility of genetic influence on such a measure looms large and becomes even more interesting as an alternative interpretation of the finding that such representations of one’s own attachment as a child relate to attachment patterns as a parent.
2.1.2.5. SIDE. Two other relevant studies involved self-reports on the Sibling Inventory of Differential Experience (SIDE, Daniels & Plomin 1985), which was developed to assess nonshared experiences of siblings in relation to parents and each other, as well as peers. Siblings rate their experiences relative to their siblings rather than in an absolute sense. For example, one of the SIDE differential parental treatment items is, “Mother has been sensitive to what we think and feel.” Each sibling answers on a 5-point scale in which 1 represents “toward sibling much more,” 3 means “same toward my sibling and me,” and 5 means “toward me much more.” The relative scoring of the SIDE can be transformed to “absolute difference scores” to assess perceived differences in experience regardless of which twin was favored. These difference scores do not permit the calculation of sibling correlations as in previous studies because the SIDE asks siblings to rate their experiences relative to their siblings.
The SIDE was used in a twin study (Baker & Daniels 1990) and in a sibling adoption study (Daniels & Plomin 1985) that compares nonadoptive siblings (biological siblings in nonadoptive families) and adoptive siblings (genetically unrelated children adopted early in life into the same family). The twin study included adult twins who responded retrospectively about the family in which they were reared, and the sibling adoption study included adolescents and young adults. If genes affect the SIDE measures, mean differences for DZ twins will exceed those for MZ twins, and adoptive pairs will exceed those for nonadoptive pairs, because the magnitude of genetic differences within pairs is in the order: MZ < DZ = nonadoptive sibling < adoptive siblings.
Table 6 lists mean absolute differences on the SIDE reported by MZ and DZ twins and nonadoptive and adoptive siblings. The twin comparisons indicate significant genetic influence both for parental affection and control and for sibling closeness and jealousy. The sibling adoption design, however, yields less evidence for genetic influence. Adoptive sibling differences are not significantly greater than nonadoptive sibling differences in their ratings of parental treatment. Ratings of treatment by one’s sibling, however, consistently show greater differences within adoptive sibling pairs than nonadoptive sibling pairs, although the difference is significant only for sibling closeness, the scale most clearly related to warmth. These are the first studies to consider genetic influence on sibling behavior toward a target child rather than parental behavior.
This suggestion of greater genetic influence in the twin data as compared to the adoption data needs to be replicated and tested for generalization to other environmental measures, it may not be coincidental that twin data for personality questionnaires also yield greater evidence for genetic influence than adoption data (Plomin & Nesselroade 1990). This finding can be explained genetically by epistatic genetic variance, higher-order interactions among genes that are entirely shared by MZ twins but not by DZ twins or other first-degree relatives who are the subjects of adoption studies. An environmental explanation has been called the MZ assimilation effect, in which MZ twins experience more similar environments than DZ twins. In personality research to date, it appears that both epistasis and an MZ assimilation effect may be responsible for twin estimates of heritability that exceed adoption studies of first-degree relatives (Plomin, Chipuer & Loehlin 1990).
For environmental measures, however, it is not yet clear that adoption data yield lower estimates of genetic influence than do twin studies. As indicated in the following section, some adoption data implicate substantial genetic influence on environmental measures.
2.1.3. Studies of children: Adoption studies. The Colorado Adoption Project (CAP) provides sibling adoption data for several types of measures. The CAP is mentioned several times in this article: It is a combined adoption/family, prospective, longitudinal study consisting of 245 adopted children studied yearly beginning at 12 months of age (Plomin, DeFries & Fulker 1988). Also assessed are the adoptees’ biological and adoptive parents, matched nonadoptive families, and younger adoptive and nonadoptive siblings. (The phrase “adoptive siblings” refers to unrelated children adopted into the same family.)
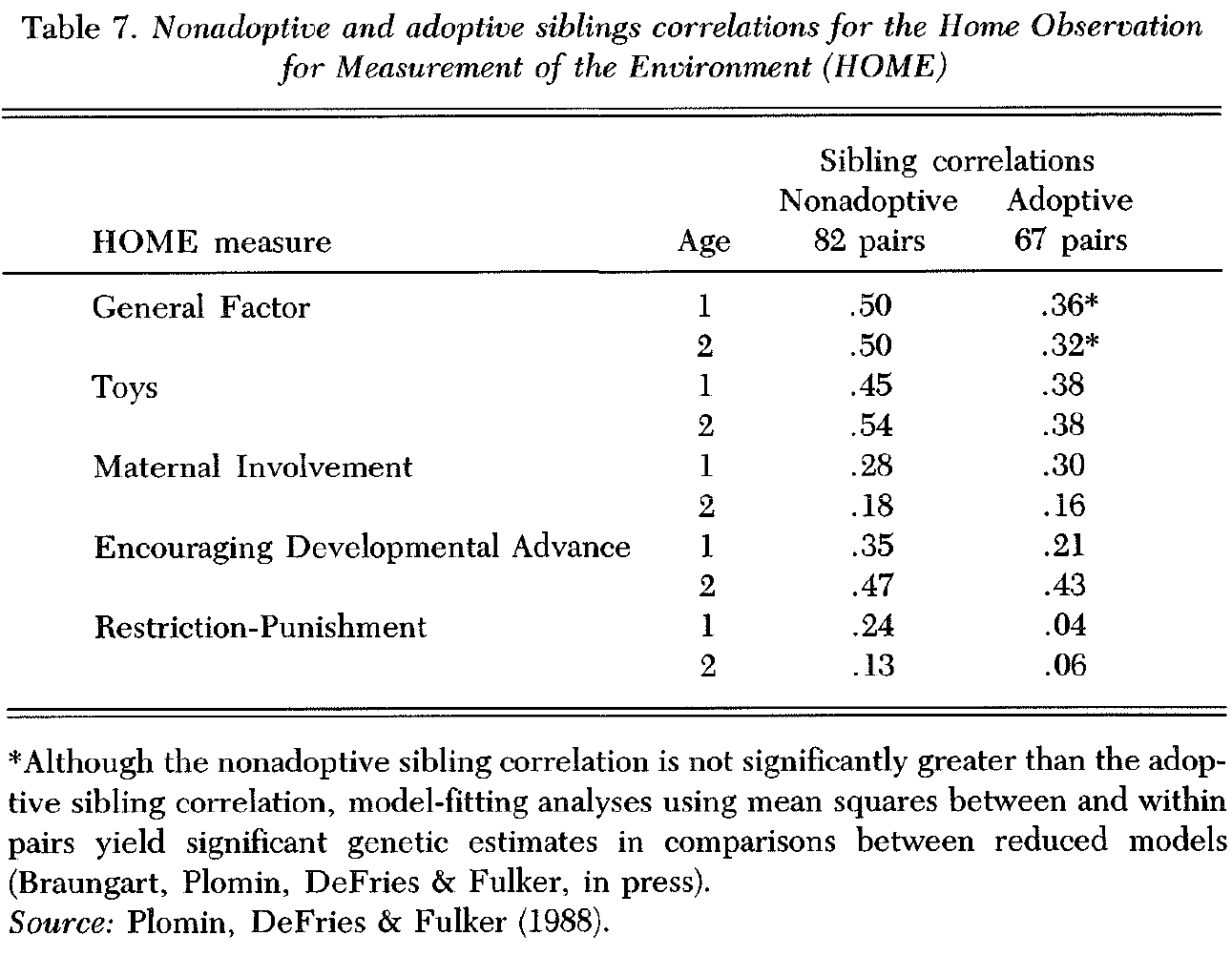
The CAP included the widely used observation/interview instrument, the Home Observation for Measurement of the Environment (HOME; Caldwell & Bradley 1978), assessed for nonadoptive and adoptive siblings when each child was 12 months and 24 months old. The HOME is problematic for detecting genetic influence on the family environment in that many of the items cannot be expected to reflect genetic differences among siblings because the items are not specific to the child. For example, such items as number of books and pets in the home will not differ for the two children and thus cannot display genetic influence in the sibling adoption design. Nonetheless, some of the HOME items are specific to each child, and these items make it possible to explore genetic influence on this objective environmental measure.
As shown in Table 7, nonadoptive and adoptive sibling correlations for the HOME general score are .50 and .36, respectively, at 12 months, and .50 and .32 at 24 months, suggesting that parental behavior reflects genetic differences among children. Model-fitting analyses confirmed this conclusion, showing significant genetic influence at both ages (Braungart et al, in press). The significant correlations for adoptive siblings indicate, not surprisingly, that the HOME also assesses environmental influences shared by siblings. The surprise is that genetic influence should count for so much for this objective measure of the home environment, especially when it is limited to parental responses to genetically influenced characteristics of the children, as discussed earlier.
Sibling correlations for subscales of the HOME suggest that evidence for genetic influence on the HOME is found for a toys scale, which assesses the number of toys of different types (e.g., challenging toys, muscle activity toys, and push-pull toys). Although the number of toys of different types might not appear to be sensitive to the particular child (because toys can be handed down from older to younger siblings) it is possible that parents buy toys for each child that reflect the child’s particular interests. The restriction-punishment results are also consistent with the possibility of some genetic influence. The sibling correlations for the HOME scale that assess encouraging developmental advances suggest genetic influence at 12 months, but not at 24 months. Maternal involvement indicates no genetic influence at either age. Although a HOME-like measure constructed for the CAP for use at 3 and 4 years showed little genetic influence, the sibling sample is much smaller at these ages and the measure is problematic in other ways as well (Plomin, DeFries & Fulker 1988).
CAP also provides the only videotaped observations of mother-child interactions that can be investigated for genetic influence. Ratings were made from videotapes of mothers interacting with each of the siblings when the child was 1, 2, and 3 years old (Dunn & Plomin 1986; Dunn et al 1986; Dunn et al. 1985). In addition to its objectivity, an important feature of this measurement strategy is that, unlike the HOME, maternal behavior specific to each child is assessed. At each age, mother and child were videotaped in three 5-minute sessions: a structured task (teaching), a moderately structured task (play with a specific set of toys), and an unstructured task (free play). Factor analysis of various behavioral counts and ratings yielded affection, control, and verbal factors. Nonadoptive and adoptive sibling correlations for these three factors at 1, 2, and 3 years of age are shown in Table 8. Despite the small sample sizes, the affection factor consistently shows nonadoptive correlations that are substantially greater than adoptive correlations. No genetic influence is suggested for the control and verbal factors, with the single exception of control at 3 years. Although the small sample size calls for caution in drawing conclusions, this is somewhat offset by the replication of results across the three years.
2.1.4. Studies of proximal family environment: Summary. Measures of the family environment show genetic influences in both twin and adoption studies, with different methods (e.g., in studies in which children or their parents rate the children’s environment), and with diverse measures of environment, including ratings, the observation/interview HOME measure, and ratings of videotape observations of mother-child interaction.
Although we hypothesized that childrearing studies of parents show greater genetic influence than studies of children, the only relevant comparison yields similar heritabilities for the two types of studies. As mentioned earlier, however, the FES used in the SATSA study in which the target was parents is not specific to childrearing. It assesses the general atmosphere of the family environment, and this could cloud genetic involvement of the respondent. More support can be found for a second hypothesis: in studies of children, parental ratings show less genetic influence than ratings by the children themselves, which is expected on the basis of the earlier discussion of components of genetic influence on environmental measures. A recurrent finding across all designs is that measures of warmth show greater genetic influence than measures of control, an unexpected finding.
Are genetic influences on environmental measures limited to measures of the proximal family environment, which may be especially caught up in the genetic concatenations among family members? The following sections review genetic research on other measures of the environment.
2.2. SES and education
Parental education and socioeconomic status (SES) are among the most widely used Indices of the home environment in studies of children’s development, and for this reason the question of possible genetic involvement in these measures should be raised. Genetic analyses of these variables require that the target sample be parents because SES and parental education do not vary for children in the family.
Both SES and parental education appear to show genetic influence, which is not surprising, given that their correlation with IQ is greater than .50 (Jensen 1980). For example, a study of 1,900 pairs of 50-year-old male twins yielded MZ and DZ twin correlations of .42 and .21, respectively, for occupational status, and .54 and .30 for income (Fulker & Eysenck 1979; Taubman 1976). An adoption study of occupational status yielded a correlation of .20 between biological fathers and their adult adopted-away sons (2,467 pairs; Teasdale 1979). A study of 99 pairs of adopted-apart siblings yielded a correlation of .22 (Teasdale & Owen 1981). All these results are consistent with a heritability of about .40 for occupational status. Years of schooling also shows substantial genetic influence; for example, MZ and DZ twin correlations are typically about .75 and .50, respectively, suggesting that heritability is about 50% (e.g., Taubman 1976). Recent SATSA analyses confirm these findings of substantial genetic influence on occupational status and years of education (Lichtenstein & Pedersen, in press), as does an analysis of Norwegian twins, which also suggests that IQ is to some extent responsible for genetic variance in occupational status and years of education (Tambs et al. 1989).
2.3. Television viewing
Time spent watching television by children could be viewed as a measure that depends directly on the child’s own behavior and thus is not really an environmental measure. Children’s television viewing has been used as an environmental measure in thousands of studies that investigate the consequences of television viewing (Pearl et al. 1982). Despite the huge research effort to investigate its consequences, little is known about the causes of individual differences in children’s television viewing (Bryant 1990). It is not merely a function of parental restrictions – 70% of parents put no restrictions on the amount of time their children watch television (Lyle & Hoffman 1972) – which makes it more plausible to consider characteristics of children, including genetically influenced characteristics, among the provenances of this measure.
Individual differences in the amount of television viewing in children were investigated as part of the CAP (Plomin, Corley et al. 1990). Both the sibling and the parent-offspring adoption designs yielded evidence for significant genetic influences. For example, the correlation for amount of television viewing in early childhood for nonadoptive siblings is .48, whereas the correlation for adoptive siblings is only .26, suggesting substantial genetic influence.
2.4. Peers
Peers represent a potentially important category of extra-familial environmental influence. The SIDE measure of nonshared environment includes three scales that assess characteristics of peer groups. The twin and sibling adoption studies described earlier (Baker & Daniels 1990; Daniels & Plomin 1985) suggest that these peer characteristics show substantial genetic influence, as indicated in Table 9. These SIDE peer scales suggest even greater influence than the SIDE parental and sibling scales (see Table 6).
2.5. Social support
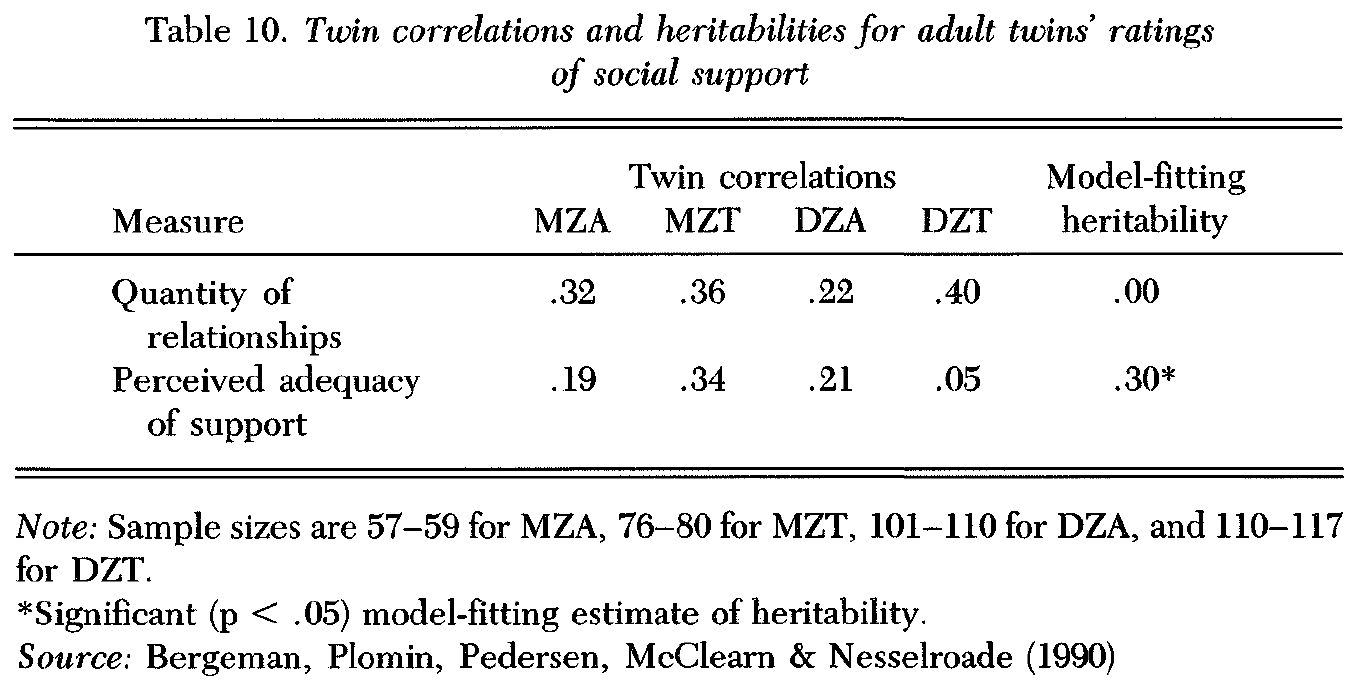
SATSA data suggest that measures of social support involve genetic influence (Bergeman et al. 1990). Nine items from a modified version of the Interview Schedule for Social Interaction (ISSI; Henderson et al. 1980) were administered. Two scales were analyzed: quantity and quality (perceived adequacy). Twin correlations, listed in Table 10, estimate significant genetic influence for the quality scale, but not the quantity scale. Although twins are similar for the quantity measure, DZ twins are nearly as similar as MZ twins on average, indicating correlated environmental influence. Model-fitting heritability estimates are 0% for the quantity measure and 30% for the quality measure.
2.6. Life events
The most recent discovery of genetic influence on environmental measures involves a widely used class of measures, life events. A measure of life events based on the Social Readjustment Rating Scale (Holmes & Rahe 1967), used in more than 1,000 studies (Holmes 1979), was modified for older Individuals in the H-70 study in Gothenburg, Sweden (Persson 1980), and included in SATSA (Plomin, Lichtenstein et al. 1990). Considerable disagreement exists concerning the best way to assess life events, and there is dissatisfaction with such traditional questionnaire measures as the Social Readjustment Rating Scale (e.g., Paykel 1983). The value of this research does not rest on its use of a particular measure of life events, however, because its goal was merely to assess genetic influence on a standard measure of life events used in many studies. Knowing that other measures might yield different results is a possibility that offers an obvious direction for future research in this area. A traditional total life events score was constructed by summing each reported event weighted by the average importance assigned to the event by all individuals who completed the questionnaire. In addition to the total score, scales were constructed to address the possibility that distinctions between controllable events (e.g., serious conflicts with child) and uncontrollable events (e.g., serious illness in child) may be important (Thoits 1983).
The twin correlations and model-fitting estimates of heritability are listed in Table 11. For the total life events score, the correlation for MZA is .49, suggesting significant and substantial genetic influence. The patterns of correlations for all four groups of twins were consistent with a hypothesis of genetic influence; the model-fitting estimate of heritability is 40% for the total life events measure.
The distinction between controllable and uncontrollable events appears to be important: The correlations for MZA are .54 for controllable life events and .22 for uncontrollable events. Maximum likelihood model-fitting analyses yielded significant estimates of genetic influence for all of the scales, but the heritability estimates are 43% for controllable events and 18% for uncontrollable events.
3. Summary and implications
Figure 2 summarizes the magnitude of genetic influence on environmental measures for those studies that permit heritability estimates. Heritabilities are plotted in relation to a dimension of presumed subjectivity/objectivity of the measures. Although this is meant only as a first rough attempt to classify environmental measures that have been used in genetic studies, there is likely to be little disagreement concerning the general ordering of measures along the subjective-objective dimension. For example, ratings of videotape observations seem more objective than interviews concerning the amount of television viewing. The latter appear to be more objective than self-report ratings of social support, and these seem more objective than self-report questionnaires about the warmth of the family environment.
An interesting feature of the results summarized in this manner is that genetic influence does not appear to be limited to subjective environmental measures. This finding suggests that genetically influenced characteristics of individuals responsible for genetic influence on environmental measures extend beyond the subjective processes involved in self-report ratings. Genetic influence appears to be not just in the eye of the beholder, but also in the behavior of the individual.
It should be emphasized again that these results by no means imply that the variance of environmental measures is entirely genetic in origin. Indeed, these data suggest that nongenetic factors are primarily responsible for variance on environmental measures. Moreover, as always, much more research is needed. The initial research on the nature of nurture that we described involves a hodgepodge of measures and a smattering of ages. More specifically, gender differences have not yet been given adequate attention, primarily because samples are not large enough to detect genetic differences between the genders.
Nonetheless, as it stands, this evidence for genetic influence on environmental measures challenges the reasonable assumption that measures labelled as environment are in fact measures of the environment. Indeed, these findings suggest that environment measures often show as much genetic influence as do measures of such behavior as personality. Even though these are very early days in research on the nature of nurture, the results would so far seem to shift the burden of proof to those who continue to assume that environmental measures are free of genetic influence.
Developmentalists have considered the issue of the direction of effects in socialization (Bell 1968); evidence for genetic influence on measures of the family environment can be assimilated in this context. Wachs and Gruen (1982), for example, have emphasized orgaeismic specificity, by which they mean that environmental influences are mediated by the organism. It must be said, however, that the issue of the direction of effects receives far more “lip service” than actual research. Also, reckoning with genetic influence on measures of the family environment goes beyond the effects of child characteristics on parents’ childrearing. For example, genetically influenced characteristics of parents can contribute to genetic influence on environmental measures independently of characteristics of the children. These findings are likely to have the greatest impact on areas of environmental research other than proximal measures of the family environment because the possible contribution of organismic characteristics has rarely been broached in these areas. For example, to our knowledge the huge literature on life events has never considered the possible role of genetic influence.
One direction for research on the nature of nurture is to continue to sort out the relative magnitude of genetic influence on environmental measures. For the field of behavioral genetics, it is interesting that genetic research on environmental measures holds out the hope that some measures are substantially influenced by genetics and others are not, unlike research on personality, for example, where nearly all dimensions show moderate genetic influence (Loehlin 1982). Research in this vein may prove useful in a practical sense in identifying environmental measures that are relatively free of genetic influence. Although heritability does not imply immutability, environmental measures free of genetic influence would seem more likely to show effects of intervention, and they would permit more straightforward interpretations of environmental influence in other research using measures of the environment.
In addition, sorting out the extent of genetic involvement for diverse environmental measures might provide clues as to the mechanisms of genetic influence. For example, why does parental affection show greater genetic influence than parental control? Why does the quality of social support show greater genetic influence than the quantity of support? Why do ratings of controllable life events show greater genetic influence than uncontrollable life events?
Such questions as these lead to what will surely be a major direction for research at this interface between nature and nurture: exploration of the processes by which genetic influence affects measures of the environment. As discussed in the introduction, environmental events have no DNA; genetic effects on environmental measures must be the result of covariation with genetically influenced characteristics of the individual. We suggest that it might be useful to consider the degree of genetic influence on an environmental measure as an index of the extent to which characteristics of the organism are assessed by the environmental measure. But this does not take us very far toward identifying the specific processes by which genetic influence emerges in analyses of environmental measures, because any genetically influenced characteristic of individuals can contribute to genetic influence on measures of the environment. Furthermore, the criterion that these organismic characteristics should be heritable is not particularly helpful, because nearly all such characteristics are moderately heritable, for example, those most often studied by behavioral geneticists: cognitive abilities, personality dimensions, and mental disorders.
The first step in this direction is to explore behavioral correlates of environmental measures. Few analyses of this type have been reported, but the results so far do not promise that traditional dimensions of behavior can account for genetic influence on environmental measures (Plomin 1986). For example, genetic influence on the HOME might be thought to result from parental IQ. The HOME correlates only .13 with parental IQ in CAP, however. The HOME correlates at about the same level with extraversion and neuroticism. Other associations between behavioral and environmental measures are reasonable but weak. For example, the FES second-order factor of personal growth correlates about. 20 with several major dimensions of personality — emotionality, activity, sociability, extraversion, and neuroticism, for example. SIDE scales also yield significant correlations with personality; the most heritable SIDE scale involves peers, and this scale correlates significantly but modestly with fearfulness, shyness, sociability, and activity. We have conducted analyses in SATSA of the personality correlates of life events and again find only modest correlations. The controllable life events score correlates only .06 with neuroticism and .12 with extraversion; the highest correlation (.21) for uncontrollable life events was found with a sensation-seeking scale.
Although these patterns of correlations between behavioral measures and environmental measures are weak, it is possible that in concert they can begin to account for genetic influence on environmental measures. These are just phenotypic correlations, however, and do not demonstrate genetic mediation between behavioral and environmental measures. Multivariate genetic analyses of the phenotypic covariation between behavioral measures and environmental ones are needed to determine the extent to which such behavioral measures can account for genetic influence on environmental measures. To our knowledge, no such multivariate analyses have been reported in which an environmental measure is analyzed as a phenotype.
There are three reasons, however, why we do not expect such multivariate analyses of genetic overlap between behavioral and environmental measures to yield simple answers. First, answers to the question of processes that mediate genetic influence on environmental measures are likely to differ as a function of method, source, and target. For example, it seems reasonable to expect that different genetic processes are involved in genetic influences on objective and subjective measures. For subjective measures, genetic influence can accumulate as ratings are filtered through the feelings, personality, and cognitions of individuals. This issue can be addressed by multivariate analyses of the genetic covariance between environmental measures.
Second, even if multivariate genetic analyses uncover genetic correlations between environmental measures and traditional dimensions of behavior, a genetic correlation is just a correlation, and does not prove that genetic influence on the environmental measure is epiphenomenal to the genetic influence on the behavioral measure. Indeed, a hypothesis that interests us goes the other way around: Genetic influence on the ways organisms interact with their environment might be responsible for the ubiquitous genetic influence found for behavior. This is the essence of Scarr & McCartney’s (1983) developmental theory of how people make their own environment. Only longitudinal analyses can begin to disentangle such questions of cause and effect.
Third, traditional dimensions of behavior may show few important genetic associations with measures of the environment because of the possibility that environmental measures extract genetically influenced patterns of reactions of individuals to their environment that are not tapped by our traditional measures of behavior. With respect to family environment for example, traditional measures of personality and cognition designed to be context-free seem unlikely to capture entirely the genetically influenced concomitants of the intense, emotion-laden context of family relationships. Attempts to assess context-specific behavioral dimensions may be more fruitful and could lead to new insights about behavior at the interface between nature and nurture. We suggest that behavioral genetic studies of attributional processes may be useful in this regard.
Finally, in addition to broaching the topic of the antecedents of genetic influence on environmental measures, multivariate analyses can be used to address their sequelae. That is, given that both environmental and behavioral measures are influenced genetically, it is possible that associations between environmental measures and behavioral outcomes are also mediated genetically. For example, if measures of life events are heritable, associations between measures of life events and psychopathology might be mediated in part by genetic influences shared by the two domains. Multivariate genetic analyses are also appropriate to assess genetic covariance between environmental measures and outcome measures, although the same caveat is in order: A causal direction from environment to outcome cannot be attributed to genetic correlations between environmental measures and outcome measures. For example, a multivariate analysis of the HOME and children’s IQ using CAP nonadoptive and adoptive sibling data found little evidence for genetic mediation of the phenotypic association between HOME and children’s IQ (Braungart et al., in press). We are aware of no other published reports of multivariate genetic analyses in which an environmental measure is treated as a phenotype. As mentioned in the introduction, however, parent-offspring model-fitting analyses of IQ have incorporated environmental measures as indices of IQ-relevant environmental variance; these studies have found some evidence for genetic mediation of the link between environmental indices and IQ.
Success in all of these research directions could be facilitated by the development of more sophisticated measures of the environment. For example, in relation to family environment, measures are needed that are specific to a child rather than general to a family. We also need better measures of experience (the subjective, experienced environment) in contrast to measures of the objective environment. Most important, we need measures that move beyond the passive model of the individual as merely a receptacle for environmental influence to measures that can capture the individual’s active selection, modification, and creation of environments – this lies at the heart of the interface between nature and nurture.
In summary, it is remarkable that research reported to date, using diverse measures and methods, so consistently converges on the conclusion that genetic influence is significant and substantial on widely used measures of the environment. This finding has far-reaching implications for environmental studies of the behavioral and brain sciences; the bottom line is that labelling a measure environmental does not make it environmental. Nonetheless, this is only a first step in a sprawling, unexplored land. Much remains to be learned, for example, about the degree of genetic influence on the many facets of environmental influence, about the antecedents and sequelae of genetic influence on environmental measures, and about the developmental course of the nature-nurture interface. Our motivation in writing this target article was to provide a solid foundation for this new field of research by documenting the evidence for genetic influence on environmental measures with the hope that this will stimulate further research on the nature of nurture.
Open Peer Commentary
To nurture nature
Diana Baumrind
All levels of biological organization and behavior have a genetic basis; this is expressed, however, only through interaction with the environment during development. If from the moment of conception each child’s nature helps to determine his nurture, and each child’s nurture helps to determine his nature, then the analysis of variance of an additive model cannot adequately represent the underlying reality it purports to explain. If all behavioral dispositions are a product of a process influenced by gene-environment (G-E) interaction, then none of the relationship between G and E can be additive, and it is not meaningful to calculate what proportion of a child’s aggressive behavior, or of a parent’s warm behavior is due to his genes or to his environment. Any heritability model that does not represent accurately how heredity and environment jointly contribute to behavioral dispositions is a bad model. If the attempt to compute heritability coefficients for human behavioral traits is inherently flawed, as many critics believe it is, it will not benefit appreciably from the refinement proposed in Plomin & Bergeman’s (P&B) target article, that G as well as E contribute to the environmental measure. [See also Wahlsten: “Bias and Sampling Error in Sex Difference Research” BBS 11(2) 1988.]
What is the social and scientific significance of assessing the extent to which genetic factors affect measures of the human environment? Surely the purpose is not to guide a eugenics program or to predict the results of selection. Is it then to discourage environmental manipulations when the heritability index is found to be high? Knowledge of the heritability of some trait in a population does not provide an index of the efficacy of a properly targeted intervention effort in altering a trait, however (Levins & Lewontin 1985; Morton 1974). Even inborn errors of metabolism with a heritability coefficient of 1.0 are completely curable by environmental intervention. Indeed, the purpose of compensatory programs to remediate deficiencies in intelligence or social skills is to alter radically the normal range of environments for those individuals to improve the phenotype. The decision as to how strenuous that environmental intervention should be must rest on ethical considerations of social justice and compassion, and not on inferences about malleability drawn from a heritability index.
In addition to general skepticism about the view of reality underlying developmental behavioral genetics, I have questions about the logic of the research design, the validity of the measures used to assess environmental influences, and the vigor with which its assumptions have been probed and corrections made for restricted range, selective placement, and unequal environments. Should readers assume, even where it is not stated, that in all studies reviewed by P&B the extent to which twins or adoptees and their caretakers differ in means and variances from the population at large has been assessed, that DZ twins are of the same sex, that the magnitude of the hereditability index is not sex-differentiated, and that corrections have been made for violations of the assumptions of equal environment and nonselective placement?
Although heritability designs generally use twins and adoptive families, the results are generalized to the normative family composed of neither. But twins are known to differ from singletons as neonates and in language learning; and caregivers are likely to treat both twins and adoptees differently from singletons or biological offspring. For example, maternal attachment may be activated more readily by the relatively nonstressful condition of nurturing a newborn singleton than by any other condition.
To what extent does the adoptive status affect parents’ behaviors? I would expect parents to be more securely attached to natural than to adoptive offspring and to vary more in the degree of warmth expressed to adoptive than to biological children. If so, the lower correlations for the HOME general score for adoptive siblings may well be the result of the effect on parents of the status (adoptive or nonadoptive) of the child, rather than the effect on parents of the greater genetic differences among adoptive siblings.
On the face of it, both the equal environment and nonselective placement assumptions in twin and adoptive studies, respectively, appear untenable. Parents treat MZ twins more similarly than DZ twins, and MZ twins choose to dress more similarly and spend more time together. Thus, Rowe (1981) reported that the violation of the equal environments assumption could account for the greater similarity of MZ than DZ twins on acceptance-rejection. P&B do not mention this violation, but conclude instead that Rowe demonstrated a substantial genetic influence on acceptance-rejection. They acknowledge “unequal environment” in the Lytton study, but attribute it to genetic influences on the caretakers when it can just as well be attributed to the MZ assimilation effect, an environmental influence. The adoption design assumes that genetically related individuals reared apart are placed in different environments. Selective placement in adoption studies (for example, separated MZ twins with high IQ adopted by parents with high IQ) would underestimate the contribution to phenotypic similarity (for example, academic performance) of E (intellectual stimulation) contrasted with shared G (high IQ). But the families in which reared-apart siblings, especially twins, are placed are likely to be selectively similar, an environmental factor that could inflate the heritability index, for example, of traditionality. On a scale from 1 to 10, do the adoptive environments differ as much as a middle-class family living in Israel does from a peasant family living in the Peruvian Andes, or only as much as 2 middle-class families living in the same town do on such parameters as SES, healthy habits, pattern of child-rearing, intellectual stimulation, and control?
Finally, the majority of environmental measures are based on abbreviated, retrospective self-report measures, long since discredited as objective measures of family influence. Although it is interesting that adult DZ as well as MZ twins agree that as children they were exposed to a similar type of parental control, and that MZ twins agree more than DZ twins in their memories of parental warmth, these retrospective memories should not be confused with actual measures of parental warmth and control. Based on the assumption that all behavioral dispositions are influenced by gene-environment interaction, all measures of environment that assess behavioral dispositions of parents or children should by definition be so influenced to some degree. Because parental control, like other parental behaviors,’ is a behavioral disposition influenced by the behavioral dispositions of children, the absence of any genetic influence on control would seem to reflect error in the model or the measure.
The methodological defects in the research designs of heritability studies are not more serious than those of other kinds of behavioral research that partition variance. But the causal claims are greater. Behavioral scientists and lay persons alike tend to overlook defects in measurement or design when findings are presented in the form of a quantifiable index that gives the appearance of being purely objective. To a critic, the degree of assignment of phenotypic variance to E often appears quite arbitrary, and the claims to social and scientific significance, quite inflated. For example, P&B state that “environmental measures free of genetic influence would seem more likely to show effects of intervention.” Yet as P&B themselves acknowledge, heritability does not imply immutability. At best, the linear model that estimates heritability is a local analysis that pertains to the actual distribution of genotypes and environments in the particular population of twins or adoptees sampled. It may suggest how much change in the existing range is required for environmental manipulations to be effective. Outside the zone of canalization that corresponds to the range of environments that have been historically common, more or less variance may appear in even these rather peculiar twin and adoptive populations. At worst, the analysis of causes in human genetics assumes a view of the real world that is known to be false. Whereas in the real world the environment and the organism are interpenetrating rather than independent, resulting in covariance as well as interaction between genotype and environment, the model assumes independence in the generation of the phenotype. The environment-as-phenotype perspective of the target article simply points to an additional source of error.
It is my contention that the underlying model of the analysis of causes in behavioral genetics neither represents accurately nor molds constructively our view of the real world. Social and behavioral scientists are not immune to the social forces they help to shape. Our view of what is is shaped by and also shapes what we think could or should be. When social problems seem intransigent, as so many do today, scientists as well as politicians turn easily to biological explanations. The thrust of the target article (whatever the motives of its authors) is to elevate genetic determinism as an explanation for human behavior. Cultural and genetic determinism both undermine the attribution of personal responsibility to the individual as a moral agent. A better reapportionment of the genetic and environmental components of phenotypic variations seems pointless if the model is badly flawed. Genetic linkage analysis can ultimately lead to insight into the biology of disease processes such as schizophrenia, but is unlikely to contribute to an understanding of variations in attitudes or normal personality attributes. As a discipline, behavioral genetics is not anchored in accepted bodies of fact nor based on biological foundations, and so offers little to the socialization researcher, whose concern is with how to help caretakers in various socio-ecological niches to nurture nature most effectively.
Heritability of what?
Fred L. Bookstein
In this review of “genetic influence on ‘environmental’ measures,” most of the scales to which Plomin & Bergeman (P&B) refer - the FES, the HOME scale, parent-child interaction, and the like — were never intended to be dependent variables, that is, scientific explananda in respect of influence of any sort. These measures were intended instead as independent: factors placing families at risk of disorganization, modulators for studies of the effectiveness of social services, or social indicators in cross-cultural studies. They are called “environmental” not because they should be thought wholly insensitive to heritable characteristics of the parent or child (the authors cite no one in support of this absurd position), but because they are sensitive to the sort of politically feasible environmental interventions or manipulations available to a caseworker or social scientist. The “environment” of “environmental measures” has the semantics not of "environmental versus genetic," but of “environmental versus personal”: a social, not a scientific, category.
Supposing, however, that we did care whether there is a genetic basis for an environmental measure, such as TV viewing. How should we study the matter? A physicist measures an “environment” - a gravitational field, for example - in a manner partially analogous to these behavioral geneticists’ measurement of an “environmental phenotype.” A single quantity (weight) summarizes a dyad of child (probe) and family (field). When the same probe is calibrated against varying gravitational fields, its weights are found proportional to a fixed series of quantities, the field strengths. Similarly, when a variety of probes are calibrated against a single gravitational field, their weights are found to be in fixed proportion, whatever the field. It is from the invariance of these extended proportions (cf. the Eotvos experiment; Will 1986) that we infer both the existence of the physical quantity called mass and the multiplicative form of Newton’s Law. The further dependence of weight on inverse squared distance is established by yet another series of comparisons (the apple vs. the moon). [1] Note these three formal aspects of the procedure: There is a quantification of a property of the object, a separate quantification of a property of the field, and a demonstration of the appropriate (multiplicative) form by which these two quantities determine the value (weight) that is measured (see also Wahlsten, 1990, and my comment there). In the absence of the first two of these features of the experimental setting there is no way to explore a functional form, and therefore no way to claim understanding of “genetic influence” or anything else.
The literature cited so fondly by P&B - tables of twin and sibling correlations and discordances - has none of the requisite epistemological precision. We do not decompose measured “weights” (values of measures of the purported net effect of genetics and environment), rather mere correlations of these values over pairs of “probes” (twins, siblings) in various “fields” (raised together, raised apart). There is no way to retrieve heritabilities from such data without assuming the absence of interaction. But notice that the usual term for interaction has been silently omitted from the path diagram in the authors’ Figure 1; the latent variable scores are not identifiable either. In the language of path analysis (cf. Plomin et al., 1990, Figure 9.14), although we can formally generate different path coefficients for “G” and “E” as they affect the phenotype, the estimated values of these latent variables must be identical up to a scale factor. That we cannot generate two distinct trait scores from one observation follows directly from the foundations of behavioral genetics, where “environment . . . literally means ‘nongenetic’” (Plomin et al. 1990, p. 249). A trait cannot be both an error term and an observable at the same time! For instance, in the method of the SATSA studies, any aspect of parental treatment that is a socially stereotyped function of the child’s age and sex will show similarity in the treatment of twins, and even higher similarity for same-sex twins, and hence will be called “heritable,” the object of “genetic influence,” even though there need be no determinants of the behavior beyond the purely sociological.
Suppose now, however, that P&B were measuring gravitational attraction between parent and child (at a constant separation of, say, three meters). Call this quantity “attractivity.” It will be found heritable, yet what is inherited is not “attractivity,” a matter of “environment,” but mass, a property of individuals. If all parents had the same weight, then our measure of “attractivity” would be proportional to the weight of the child. Hence we would still find “attractivity” to be heritable (because weight is), although now it would have nothing to do with the “environment” at all. As parents do themselves vary in weight, however, we cannot recover from these “attractivity” data any understanding of weight or its actual heritability. The quantification of the dyad by its “attractivity” has conflated two quantities that needed independent measurement: the mass of the child and the mass of the parent. If the distance at which “attractivity” was measured is itself a function of weight, or otherwise heritable, then the disentanglement is even more complicated.
Labelling an algebraic function of twin correlations as the environmental measure’s “heritability” is not helpful in the absence of knowledge of just what is inherited, and how. By the intentionally absurd example of “attractivity” I mean to make a quite serious point. In biometrical statistics one does not claim to have measured an “influence” until one has a regression slope for it. This requires quantification of cause, quantification of effect, and a demonstration of the functional form relating the two. The literature reviewed in the target article apparently skips all of these desiderata. The very word “heritability” should not be applied to a concept like “television viewing” (Figure 2). What has this finding to do with the origin of the concept of heritability in parent-child regressions? Even if twins reared apart watch television in more similar patterns (corrected for age and sex) than siblings reared together, one must ask: Heritability of what? What is the nature of the genetic information underlying “television viewing,” what is the manner of its inheritance, and what is the manner of its interaction with characteristics of the context in which the child is observed (presence of television sets, other siblings, parental taste)? LISREL outputs (coefficients of heritability) notwithstanding, the reduced path diagram of the target article’s Figure 1 is perfectly useless as long as we lack estimates of the causes that these path coefficients multiply. The literature reviewed here is, therefore, formally quite irrelevant to the program of research proposed in section 3, the study of “genetic mediations” of correlations between environment and “traditional dimensions of behavior.” Inasmuch as no one appears to disagree with the target article’s principal claim, one wonders why it was written at all.
P&B must take their Figure 1 seriously as a directive for measurement, not mere modeling of correlations. They must show independent measures of what It Is that is Inherited and how the “environment” mediates its expression, with special reference to the form of interactions. Twin correlations over environmental measures are not useful for this program of research. They yield abstract “heritabilities,” but no quantities that are to be considered heritable, nor any evidence that the assumptions underlying the estimated heritabilities are justified. In Its attempt to use the opportunistic “environmental” measurement design as rhetorical support for the much more difficult studies of development already proceeding under other auspices, P&B’s target article eschews all rigor In respect of the notion of “genetic Influence” and misconstrues the logic of measurement in Its understanding of “environmental measures.”
NOTE
1. For an application of the identical logic in the psychological sciences, see Luce & Tukey (1964).
Implications for behavior genetics research: No shared environment left?
Dorret I. Boomsma and Peter C. M. Molenaar
As Plomin & Bergeman (P&B) themselves state repeatedly, an environmental measure is just another phenotype whose genetic architecture does not change because we call it an environmental measure. The exact amount of genetic determination of such measures is an empirical issue and is interesting, as is the heritability of different environmental variables. Equally interesting, however, is the question of what the implications are for traditional behavior genetic models, such as those used in the analysis of twin and sibling data, if environmental indices are genetically influenced. More specifically, what are the consequences if some environmental measures that are perfectly correlated within siblings or twins show an association with the trait under study? A measure that is perfectly correlated within siblings - the number of books in the house, for example - increases the environmental variance shared between siblings. Does the finding that variance in many traditional environmental measures contains a genetic contribution, as P&B illustrate in their target article, or the finding that correlations between measures of the home environment and IQ can be mediated genetically (e.g., Coon et al. 1990), imply that shared environmental variance also is not completely environmental?
In our Figure 1, the elementary model introduced by Plomin, Loehlin & DeFries (1985) to study relations between an environmental index, parental genotype, and offspring’s phenotype is extended to families with two siblings. H is the Figure 1 (Boomsma & Molenaar). Path model of the effects on sibling resemblance of a correlation (r) between parental genotypes (G-father and G-mother) and an index of home environment (H). P, E, and G are children’s phenotype, environment, and genotype (variables in squares are measured, variables in circles represent unmeasured factors). Path coefficients h, e, and f assess the direct effects of one variable on another. For simplification, the model leaves out direct parental influences on the child’s phenotype, assortative mating in the parents, and shared environment in the children that is independent of H. measured home environment (e.g., number of books) that influences the environment (E) in which children grow up. Children’s environment and genotype (G) influence their phenotype (P), a measure such as IQ. As shown by Plomin et al., the correlation between H and P equals ef + rh. Following path analytic rules, we can derive the correlation between the phenotypes of the first and second child as: ef(ef + rh) + 0.5h². The first part of this expression is the resemblance of siblings induced by the shared environmental variable H. If the trait under study is heritable and parental genotypes are correlated with H, then what emerges as common environmental influences in the children (as, for example, in an analysis comparing identical and fraternal twins) turns out to also include a genetic component!
In an earlier BBS target article Plomin & Daniels (1987) pointed out that very little shared environmental influence is usually found for measures of personality and psychopathology. The only trait showing some evidence for common environmental influences is cognition early in life. This is also the measure that correlates reasonably high with some indices of the home environment. Does the fact that H can be genetically influenced mean that there is even less room for shared environment than we already thought?
Like images refracted: A view from the interactionist perspective
Robert H. Bradley and Bettye M. Caldwell
Plomin & Bergeman (P&B) execute a bold new variation on standard quantitative genetic analyses by treating environmental measures as phenotypes. As devoted gardeners of the interactionist position in the nature/nurture plot, we find many things to admire in this approach, yet other things about which to be wary. Interactionists believe the artificial separation of genetic and environmental influences belies the operation of genetic and environmental action in human development. The evidence presented in this target article helps lay to rest the myth of an independent set of environmental forces.
What is disappointing in the discussion by P&B is their failure to adopt a clear interactionist position. Repeated use of the term “genetic influence” clouds the contribution of an interesting new approach to the nature/nurture issue. Based on information developed using quantitative genetic analyses, the term “influence” simply refers to the increased probability of some score on an environmental measure, not a genetic blueprint (Oyama 1985). Neither can one leap from population statistics to any particular “mechanism” that drives the course of individual development. As surely as environmental measures do not actually represent some set of environmental forces “out there” in reality, neither do those measures reveal a specific set of genetic directions “in there.” P&B essentially concede this point, yet they continue to use the term genetic influence; they insist that they are not really talking about environments as if they were phenotypes, but about measures of the environment as phenotypes. There may be a bit too much cleverness in this position. It isn’t always easy to keep such distinctions separate, especially when one is trying to derive practical implications from the results. Johnston (1987) presents a revealing account of how myths about genetic or environmental influence persist despite the overwhelming evidence in favor of the interactionist position.
It is all too easy to forget that methods in science are simply metaphors in action. As we begin to layer these metaphors one upon the other, risk increases that results based on the metaphors will only grossly reflect the “realities” under investigation, like images refracted in a mountain pool. Most studies cited by P&B have unrepresentative samples, and the environmental measures tend to have psychometric problems. Combine these potential sources of error and uncertainty - little robust theory to drive strong hypotheses about expected geneenvironment correlations - with failure to fulfill statistical assumptions, and one wonders at the meaning of some of the heritability estimates that emerge. It is unlikely that similar estimates of environmental measures would emerge across cultures or in some atypical populations (e.g., the severely handicapped).
P&B might find a richer research yield in the idea of environmental complicity with genetic predispositions. It is likely that many societies have long recognized individual differences and organismic complexity and that they have adapted their reactions to allow for many variations initiated by gene action. It is not just that “individuals select, modify, and even create their environments,” as P&B contend. There are actors on the other end of this action sequence who often permit, even encourage, the actions. What does this mean? It means that in many living systems there is likely to be a large measure of complicity in the environment relative to the individuals who encounter the environment. As a result, we tend to get an alignment between a person's characteristics and the characteristics of the environment in which that person lives. In such circumstances, the methods of quantitative genetics will show significant heritability. The idea of genetic influence, however, seems to lose meaning in this complicated arabesque. It is an adaptation of a person within an environment, each adapting to the other over time but with the environment allowing many traits to reach fulfillment - no argument with Scarr & McCartney’s (1983) genetic quarterback notion intended. In circumstances where a broad range of activity from the environment is impossible or not allowed, the heritability estimate for particular environmental characteristics is likely to be quite low. Genes have no more influence on environmental measures than the environments in which they operate allows.
Like Scarr and McCartney (1983), P&B note the distinction between “environment-as-phenotype and genotype-environment correlation.” Scarr and McCartney have provided a very careful analysis of the processes whereby gene-environment correlations are increased. It is a position with which P&B - and we - largely agree. We think, however, that a reexamination of twin and adoption studies that form the basis for most conclusions about nature versus nurture presented by P&B, taken together with studies that deal with extreme environments, would lead to a conclusion somewhat different from P&B’s. Specifically, both genes and environments in the middle range for a variable are relatively impotent in the company of extreme levels of the other. It is the extremes that account for the apparent power in statistical analyses. The samples for twin and adoption studies rarely include sustained extreme environments, especially negative ones. Remove the top and bottom 10% from the genetic pool, recompute the statistics, and suddenly the relative power of genes will appear to have shrunk. The perceived impact of genes on environmental measures will accordingly shrink. The evoking power of gene-driven differences is far greater at the extremes. The child who is a little overactive isn’t likely to get his parents to change their behavior much - it is the kid who is “hell on wheels.” The 110 IQ child doesn’t evoke a response from the environment much different from the one a 100 IQ child evokes: the 140 IQ kid does. Living systems don’t generally do much fine tuning in reaction to differences in the midrange (i.e., comfort zone) of most characteristics of its members; Not much pressure to change usually occurs in that range. What happens is “gross tuning” when behavior falls outside the comfort zone (e.g., the extreme underresponsiveness of very low birthweight children). Most of the apparent impact of genes on environments is the result of phenotypic extremes. It is also usually the extremes on a genotype who find their environments ill-fitting, thus encouraging them to become active in trying to make adjustments in the environment. In summary, extreme cases have disproportionate effects accounting for much of the variance in gene-environment correlations. It is at the extremes of genotypes and “envirotypes,” and especially at their clash points, that we are likely to learn most about organism/environment interactions.
Two final comments: First, freedom from “genetic influence” is not a precondition for a good environment measure, as P&B seem to imply. An effort to create “genetics free” environmental measures would probably be unproductive, capitalizing on the trivial, the accidental, and even eccentricities of distributions. P&B’s recommendation to construct more sophisticated measures of the environment is well taken, however. Our constructs are generally better than our measures.
Finally, P&B’s speculation that “environmental measures free of genetic influence would seem more likely to show effects of intervention” seems premature. It is not yet grounded on a convincing theory of organism/environment relations in living systems, and it ignores the leveraging power of extreme genes and environments on average levels of the other. A high level of sustained enriching stimulation (a genetically associated environmental circumstance) will move the majority of children in an upward direction developmentally. So few relationships like this have the status of scientific “fact”; let us not try to discredit them, even inadvertently.
The nurture of nature
Urie Bronfenbrenner
Plomin & Bergeman (P&B) present us with a provocative title, provocative findings, and a challenge to discover the connection between the two. That challenge is important, for it simultaneously calls attention both to the impressive progress that the field of human behavior genetics has achieved over the past decade (in no small part through the work of Plomin and his colleagues), and to what, in my judgment, constitutes the major obstacle that must be overcome for any further significant scientific advance. I refer to the continued reliance of researchers in this domain on what, in Kurt Lewin’s (1935) terms, represents a class-theoretical, additive model that focuses on outcomes without specification, either at the level of theory or its operationalization, of the processes through which the outcomes are achieved. What P&B justifiably call “one of the most powerful designs in the armamentarium of behavioral genetics” still yields, therefore, a product that does not go beyond a partitioning of the variance in terms of summable proportions attributable to heredity and environment, and their respective subcategories. [See Wahlsten: “Bias and Sampling Error in Sex Difference Research” BBS 11(2) 1988.] As a result, the model has not answered, and cannot, what is surely the key question in the field of human behavior genetics, if not in developmental science as a whole. To state it in its most general form: “What are the processes through which genotypes are transformed into phenotypes?” Human potentials for competence and character do not spring forth full-blown like Athene out of Zeus’s head from the blow of Vulcan’s hammer. As with every outcome of human development, such characteristics must be nurtured into being. How does such nurturing take place?
The same questions speak between the lines in P&B’s important article. In contemporary behavioral science it has become almost a truism that development is a joint, interactive function of genetics and environment. In human development there can be no environmental effects that do not involve a significant genetic component, and there can be no genetic effects that do not involve a significant environmental component. P&B present impressive new evidence for the validity of the first half of the foregoing proposition, but they do not address the nature and reality of the second.
Yet the evidence is there. It appears in a variety of forms. Among them is a phenomenon that Plomin and his colleagues were the first to demonstrate systematically on a broad scale (as evidenced by many of the findings summarized in the present paper), namely, the fact that certain human characteristics and behaviors show a higher genetic component in nonshared than in shared environments (Plomin & Daniels 1987). Since neither twins nor siblings alter their genetic characteristics when reared apart, this paradoxical pattern - to the extent that it is an objective reality rather than the product of subjective perceptions - must be a function of differences in the processes taking place in the two types of environments.
What might these differences in process be? Again, the models that P&B have used, powerful as they are, cannot tell us; all they can and do tell us is that many environmental measures have a significant genetic component. To be sure, that is important information, for it is consistent with, and argues for, a conceptual and operational model that allows for interaction between heredity and environment.
That model has yet to be stated in formal terms, but some clues to its necessary elements are provided from a review of the relevant research literature. The first clue comes from two such reviews (Bronfenbrenner 1989; in press) in which I sought to discover what is known about the mechanisms through which human potentials become realized over the course of development.
Upon examining the research bearing on this subject, I was somewhat surprised to discover that such processes are relatively few in number, at least in terms of existing knowledge. To summarize them in necessarily brief compass, they are essentially of two kinds, often operating simultaneously in synergistic fashion. Both involve progressively more complex, mutually responsive interactions with the immediate environment on a fairly regular basis over extended periods of time. In the one case, the interaction takes place (or fails to do so in sufficient degree) with other persons, at first usually older, but soon with those of the same age or even younger as well. In the other case, the interaction is with physical and symbolic features present in the particular setting that invite, and permit (or inhibit) engagement in exploring, elaborating, and restructuring the immediate environment.
The available evidence indicates further, with respect to both mechanisms, that the nature of the interaction, and its developmental effectiveness and outcome, depend in part on genetically based characteristics of the parties involved. But they also depend on objective environmental conditions and events that are, for the most part, rather independent of the genetic endowment of the individual participants engaging in the interaction. Chief among them are the systematic variations, both within and between various social groups, in the availability of time and place for the participants to get together on a regular basis, as well as variability in the social structures, living conditions, life styles, competing environmental demands, and shared belief systems that, to quote, "facilitate or inhibit processes of sustained, mutually responsive patterns of progressively more complex interaction on a regular basis over an extended period of time." [1] Consistent with the primacy of environmental conditions in this regard is a fact regrettably not mentioned in P&B's review, namely, that heritabilities for environmental measures are substantially lower than those typically obtained for measures of psychological characteristics of the person.
The foregoing ideas and findings, when juxtaposed with the results yielded by the powerful research design employed by Plomin & Bergeman, suggest a possible direction for the development of a composite model that also incorporates assessments of specific developmental processes of organism-environment interaction. That design would take as its external frame the sophisticated partitioning of variance into refined measures of genetic components of the type developed and so successfully applied by Plomin and his colleagues. But within that frame there are two additional elements. The first are measures of the relationship between, on the one hand, concrete reciprocal processes of organism-environment interaction (e.g., indices of mutual mother-infant responsiveness), and on the other hand, measures of developmental outcomes. The second new element expands the model into a stratified design in which the foregoing analysis is repeated in two or more contexts that present contrasting conditions for the functioning - and perhaps the substantive outcome as well - of interactive processes that enable the realization of human potential.
NOTE
1. Limitations of space preclude presenting the specific research findings on which this statement is based; these are documented in the above-cited reviews plus the following additional sources: Bronfenbrenner 1979; 1986; Bronfenbrenner & Crouter 1983.
Cleaning up the environment
Avshalom Caspi
Psychologists have long labored to decipher Kurt Lewin’s (1935) cryptic formula, B = f(P,E), and Plomin & Bergeman (P&B) remind us that progress has been slow. The emerging consensus among trait psychologists about the “Big 5” notwithstanding (John 1990), defining the Person is hard. Defining the Environment is harder still. And then there is the ineffable “comma.”
How should we measure the environment? P&B state their goal clearly: “to explore the extent of genetic influence on measures that are thought to he and are widely used as measures of the environment.” The evidence is equally clear in suggesting that available measures are not necessarily the best ones. Still, there are more sophisticated ways of thinking about the measurement of environments than simply ordering them on a bipolar “subjective-objective” dimension, as P&B do in Figure 2.
Consider the conceptualization of human environments offered by Moos (1973), in which he identified six methods of measuring the characteristics of situations in relation to human behavior. Like all taxonomies, this one is not without problems, but it may be instructively resurrected to clarify our thinking about how to measure “nurture.”
According to Moos (1973), environments can be analyzed in terms of their functional properties. At this level of analysis, environments are characterized by the reinforcement consequences that persons are apt to experience for particular behaviors. This level of analysis is well represented by the various “objective” measures of maternal behavior toward children that are analyzed in the target article. According to P&B, these measures are subject to substantial genetic influence.
Environments can also be compared in terms of their psychosocial and organizational climate. How much emphasis is placed on doing well in school? How much encouragement does the child receive? Such characteristics determine the atmosphere of a social setting and are well represented by the perceived climate scales (as well as the social support measures) analyzed in the target article. According to P&B, these measures also show substantial, albeit more differentiated, genetic influence.
A third level of analysis focuses on characteristics of milieu inhabitants. This may include, in addition to easily ascertainable demographic characteristics, the salient interests, abilities, attitudes, and personality traits of persons in the environment. This is not reviewed by P&B (I would group the self-report SIDE peer scales in Table 9 under the aforementioned rubric, psychosocial climate), but I suspect future research will marshal evidence that points to genetic influence on these environmental characteristics. Consider research on marriage. It is reasonable to conceive of marriage partners as environments; after all, our partners define the stimuli and reinforcements to which we are subject for many years (Buss 1984). Studies of families of twins may well show that the spouses of MZ twins are more similar than those of DZ twins. Measures of the marital environment could then be said to have a genetic influence. Consider research on peer relationships, as well. The peer group is certainly an important environment; children’s social networks define norms for tolerable conduct and play an important role in the consolidation of behavior patterns. More often than not, individuals select companions who are similar to themselves (e.g., Billy & Udry 1985; Kandel 1978; Newcomb 1961). Moreover, they do so with regard to heritable attributes. Aggressive children tend to affiliate with friends who match their antisocial behavior (Cairns et al. 1988). Early-maturing girls tend to befriend other early-maturing girls (Stattin & Magnusson 1990). If future twin studies assess characteristics of peers, these measures may well indicate genetic influence, as suggested by Rowe and Osgood (1984) in their study of delinquency and association with delinquent peers.
According to Moos (1973), there are three additional methods by which characteristics of the environment can be related to behavior. Organizational structure dimensions refer to structural characteristics of settings (e.g., schools) such as population density, teacher-student ratio, and teacher continuity. Behavioral settings are characterized by the spatial and social properties that regulate activities among group members. And finally, ecological dimensions refer to various geographic, meteorological and man-made features of our environment. These measures of the environment are more likely to be immune to genetic influence. [1]
As this brief makes clear, there is little agreement about which aspects of the environment should be analyzed. In fact, there is little agreement about what level of analysis should be emphasized. P&B cannot be faulted for this, of course. Rather, their work serves to expose our impoverished approach to environmental assessment. Unfortunately, the choice that is usually offered between the environment as it is perceived and the environment as it is defined by objective properties is unsatisfying (Block & Block 1981). On the one hand, perceptual measures of the environment can be tautological: One can only know individuals by watching them behave in a situation, but one can know the situation only by watching the individual behave in it. On the other hand, the physicalist approach does not express adequately the prescriptive and proscriptive significance of situations to the individual. The Blocks (1981) point to a third, more promising approach: Assessments of situations can represent “the stimulus context as generally understood and, therefore, as it ‘should’ register or be understood by any individual” (p. 87).
Thus, Arsenian and Arsenian (1948) have shown that it is possible to define the formal properties of “tough” and “easy” cultures; the Dictionary of Occupational Titles describes demand characteristics that underlie most occupations in our culture, and the Blocks (1981) have been able to describe problem-solving and cognition-demanding situations in terms of their normative meaning. In this spirit, Bern and Funder (1978) also offered their template-matching technique, which they proposed to describe the personality of environments. Instead of describing environments in perceptual or physical terms, they sought to describe situations in terms of how several ideal, imaginary types of persons would function in those settings.
Whatever the problems and promises of these various alternatives, they share an assumption that is reinforced upon encountering P&B’s findings: We need to keep person and environment variables “pure” and distinct from each other if we ever hope to integrate them explicitly in an interactional theory of personality and behavioral development (Bern 1983; Caspi & Bern 1990).
The ineffable “comma” or: What is the source of the genetic influence on environmental measures? It is by now widely acknowledged that behavior is shaped in large measure by interactions between the person and the environment. There are many kinds of interaction, but there are two that may play an especially important role in generating the documented genetic influence on environmental measures.
Reactive interaction occurs when different individuals exposed to the same environment experience it, interpret it, and react to it differently. An anxious, sensitive child will experience and react to authoritarian parents in very different ways from a calm, resilient child. The person who interprets a hurtful act as a product of malice will react differently from one who interprets the same act as the product of incompetence. Each individual thus extracts a subjective psychological environment from the objective surroundings, and that subjective environment is what shapes subsequent social commerce. This is the basic tenet of the phenomenological approach historically favored by social psychology and embodied in the dictum that if people “define situations as real, they are real in their consequences” (Thomas & Thomas 1928).
The process of reactive interaction may account for genetic influence on subjective measures of the environment, but it fails to explain the genetic influence on SES, television viewing, and life event measures. A proactive process is more likely to be operating here. As noted in Scarr & McCartney’s (1983) developmental theory of gene-environment correlations, individuals select and create environments of their own. Moreover, this dispositionally guided selection and creation of environments becomes increasingly influential in development as the person gains increased autonomy from the imposed environments of childhood. P&B accurately anticipate the importance of longitudinal studies in addressing this process.
Consider the work environment. Longitudinal studies suggest that a social selection effect may account for genetic influence on environmental measures of adult occupational status. One example comes from Kohn & Schooler’s (1983) ambitious longitudinal work on personality and the workplace. Men with intellectual flexibility and self-directedness move into complex jobs; the complexity of their work then continues, in turn, to enhance their flexibility and self-directedness. Our own research on the continuity of interactional styles across the life course also suggests that personality dispositions in childhood are related to socialization environments in adulthood (Caspi et al. 1989). In one study, we examined men who had been explosive and ill-tempered in late childhood. The major finding was that those boys who came from middle-class homes suffered a progressive deterioration of socioeconomic status as they moved through the life course. They were somewhat more likely than their even-tempered peers to terminate their formal education early; the occupational status of their first jobs was significantly lower, and by mid-life their occupational status was indistinguishable from that of men born into the lower classes (Caspi et al. 1987). In summary, both reactive and proactive person-environment interactions conspire in “the nature of nurture.”
As Plomin and Daniels did in 1987, Plomin and Bergeman demand that we suspend our assumptions, reevaluate our strategies, and rethink our future directions.
NOTE
1. Social class cannot fit neatly into one of the six categories proposed by Moos (1973) because it shares a little with each category. As students of social structure and personality have noted, the first step in analyzing macrosocial phenomena is to examine their multiple components (House 1981).
Genetic effects on “environmental” measures: Consequences for behavior-genetic analysis
Wim E. Crusio
Dr. Plomin is one of the most accomplished human behaviorgeneticists of the moment, as evidenced by his many valuable contributions to the field and those of his collaborators. Again, the present target article has some important implications, this time not only for behavior genetics, but also for mainstream psychology. Plomin & Bergeman (P&B) argue quite convincingly that certain measures often used by psychologists to assess environmental influences on a subject contain a genetic component. Hence, at least as long as indirect environmental measures are involved, these variables are not valid as such, but, rather, as phenotypes amenable to genetic analysis. Unfortunately, P&B do not phrase their conclusions in this way. Although in their title the word “environmental” is placed in quotation marks, in the rest of their target article they stick to this terminology. In my opinion, this is unfortunate and promotes confusion between some important concepts of genetic analysis.
In their introduction, P&B explain the difference between a so-called environmental measure and environmental influence. I would have preferred if from this point on, they had distinguished more clearly between the different types of so-called environmental measures. It is evident that many measures labeled “environmental” are, in fact, variables that at least partly reflect properties of the subjects themselves (such as ratings of the environment). Treating such variables as environmental measures is clearly invalid.
A more disconcerting implication of P&B’s findings is not elaborated upon in the target article, however. I would like to illustrate this aspect with P&B’s example (also in the Introduction), in which the number of accidents experienced by a subject is taken as an environmental measure. As stated by P&B, this measure may be influenced by such personality traits as risktaking tendency. If the latter were influenced genetically, this would mean that people carrying particular alleles would, in general, experience more accidents than persons carrying other alleles. Unlike other “environmental” measures like parental care (which is a characteristic of a subject’s relatives) or perceived parental care (which is the same as the foregoing, but now filtered through the subject’s own personality), the number of accidents experienced may indeed be considered a direct measure of environmental influence. This is therefore a prime example of genotype-environment covariation, cov(G,E): Subjects of some genotypes will prefer high-risk environments more often than those of other genotypes. Measures that reflect (possibly genetically influenced) properties of subjects’ relatives (mostly their parents), with whom they share part of their alleles, may represent other cases of cov(G,E).
Insofar as all “environmental” measures discussed by P&B do indeed reflect some properties of a subject’s environment, the target article may be interpreted to show that genotype-environment covariation plays a pervasive role in the regulation of human behavior. Whereas in animals cross-breeding experiments may be designed in ways that minimize cov(G,E), this cannot be done in human populations. Still, almost all quantitative-genetic designs that are applicable to human populations assume that cov(G,E) is absent or negligible. If cov(G,E) is indeed present, the results obtained using such designs will be biased in rather unpredictable ways. Furthermore, even when disregarding this problem, any genetic effect now becomes very difficult to interpret: It may be direct (that is, the phenotype is the direct result of the expression of certain pieces of DN A) or indirect (e.g., when a phenotype is influenced mainly by environmental influences, but the subject shows some genetically influenced preference for certain types of environments). In my opinion, the most important aspect of P&B’s findings therefore lies in its consequences for the interpretation of a large body of human behavior-genetic research.
On the misuse of certain concepts derived from genetic analysis
M. Duyme and C. Capron
We address ourselves to two issues in this commentary. The first concerns the misuse of certain concepts in human genetic analysis. The second is relative to the measure of SES (socioeconomic status).
The misuse of certain concepts. (a) Phenotype. The phenotype is defined as the appearance (or the characteristics) of an organism that results from the interaction of its genetic constitution with the environment (Lewin 1987). The use of the term “phenotype” should be limited to those traits for which genetic correlates have been identified. As Plomin & Bergeman (P&B) themselves point out, an environment cannot be considered to be a “phenotype,” since an environment has no genes. “Sensu stricto,” the same can be said for measures of the environment.
The aim of P&B’s article is acceptable if defined as the study of similarities among relatives for measures “that are thought to be and are widely used as measures of the environment” from which behaviors and biological variables may be inferred. Evidence of these resemblances might then lead to the study of the genetic influences on different behaviors among individuals or on certain biological characteristics. It would make it possible to propose hypotheses about the variables that are the target of genetic analysis.
A question remains as to the usefulness of this step in genetic analysis. The answer depends on one’s objectives. If the object of genetic analysis is not the measures of the environment, but the behaviors contributing to the environment, then the answer is yes. Consider the example of burrowing by the wildhouse mouse. Bouchard and Lynch (1989) found that there were marked differences within, but not between populations and that members of full-sib families built qualitatively and quantitatively similar burrows. Genetic analysis demonstrated a high heritability of burrowing behavior. In this study, the authors set out to measure burrowing “to attempt to quantify this behavior in a way that makes the data more tractable for genetic analysis” (p. 448). Burrowing is defined as “the construction of a subterranean network of tunnels and/or chambers, with one or more exits to the surface” (p. 448).
This example is instructive for two reasons. First, the authors did not study the genetic influence on environmental measures, but on behaviors that resulted in the creation of an environment. Second, in their study, the exact estimation of heritability was not an aim in itself, but a means of determining whether or not screening for this trait would be effective. The relevance of this type of study is unquestionable. On the one hand, it makes possible the genetic analysis of the differences in behaviors that underlie the measured environments; on the other, this kind of study may lead to better successive experimental designs for localizing and isolating genes.
Is it possible to transpose this kind of analysis to humans to study any environmental variable whatsoever? For example, if one studies the genetic influence on measures of human habitat, what exactly is one measuring? The behavior of the owners? the architect’s design? Local architectural and judicial norms? A measure of the environment may be submitted to genetic analysis only if this measure is a reflection of behaviors for which it might be possible to localize the genes.
(b) Heritability. Although numerous authors have pointed out that estimates of heritability are of little relevance to human genetics, P&B use them to evaluate the influence of genetic factors and to compare different studies. The numerous biases inherent in the use of such estimates as applied to human beings are well known (see Roubertoux & Capron, 1990, for a recent review). Even if one were to deem such estimates free of bias, it would still be senseless to compare values having different genetic meanings. In fact, the estimates of heritability obtained have different meanings depending upon their source: studies of twins (which are designed to estimate broad heritability) or adoption studies (which aim to estimate narrow heritability). A given trait for a given population may have a high degree of broad heritability and a much lower degree (approaching zero) of narrow heritability. P&B find themselves directly confronted with this problem in the SIDE study. Moreover, since the estimates may be calculated using different models and different assumptions, such estimates give rise to different heritability values for the same data. This has been the case in studies based on either the adoption method (Horn et al. 1979) or the twin method (Vogel & Motulsky 1986). Two identical heritability values do not, therefore, imply that phenomena are identical; in the same way, two different values do not indicate that genetic factors have a different effect.
P&B suggest that a low degree of heritability for measures of the environment would indicate that the latter are “free of genetic influence.” It is a well established fact, however, that a given trait may benefit from a high degree of heritability in one population and a low one in another. In other words, even if one were to accept P&B’s point of view, this would in no way undermine the notion that a measure of the environment may be “free of genetic influence” in one population while remaining “unfree of genetic influence” in another.
Measure of SES. P&B have suggested that the aim of their research is to take “a first step in a sprawling unexplored land.” One might ask oneself whether it is a truism or a recent discovery to have shown that there are similarities among relatives on measures of environment. If one takes the example of SES, this land is not unexplored. Indeed, in the latter half of the nineteenth century, Galton (1869) and the Eugenicists largely developed the idea that the economic and social status of an individual was a function of his “heriditary value.”
This idea is equally present in a much earlier period, and can be found in the works of Plato (The Republic). As early as 1913, Richardson proposed to study adopted children to dissociate what was passed on by biological parents from what was transmitted by the postnatal environment. What is new, in our opinion, is the scientific evidence (see the work of Teasdale & Owen as cited by P&B). What is also new is the refinement of the adoption method, which presently gives rise to experimental designs close to those used for induced experimentation (Capron & Duyme 1989). What seems equally new, is that (a) it is now known that the adoption method does not allow one to dissociate genetic from prenatal factors, and (b) thanks to experiments conducted on animals, there is evidence of the effect of prenatal factors on variations in nonpathological behaviors (see Roubertoux et al., 1990, for a recent review). In the absence of more complete information on the effects of the prenatal environment in humans at the present time, studies of adoptees alone are unable to demonstrate clearly the influence of genetic factors on any variable, and even less so on environmental variables.
Finally, P&B must be thanked for having brought together in this article under a single research heading, various studies concerning similarities among relatives on measures of the environment. Some studies pertain to objective measures, others to representations. It remains to be determined whether these representations are truly indicators of environmental variables.
Parental criticism and warmth toward unrecognized monozygotic twins
Robert Goodman and Jim Stevenson
The evidence cited by Plornin & Bergeman (P&B) draws heavily on classical twin studies reporting that monozygotic (MZ) twins who are reared together typically experience (or report) more similar environments than same-sex dizygotic (DZ) twins who are reared together. The key question is, what accounts for this MZ/DZ difference? There are two radically different answers. The genetic theory, espoused by P&B, assumes that genes influence the ways individuals help shape their own environments, and that MZ/DZ differences reflect the different degrees of genetic relatedness. Alternatively, these MZ/DZ differences could be due to zygosity stereotyping, with parents and others responding more equally to “identical” than to “nonidentical” twins for purely cultural reasons. Could zygosity stereotyping be the whole story? P&B marshall two main lines of evidence that this is not the case. First, adoption studies (whether of singletons or adopted-apart twins) support the findings from classical MZ/DZ comparisons. Second, observations of parent-twin interactions suggest that MZ/DZ differences are greater when the interaction is initiated by the child rather than by the parent.
We wish to expand on a third line of evidence mentioned only briefly by P&B, deriving from the study of MZ twins who are believed to be “nonidentical” by their parents and others. Twins and their parents are commonly mistaken about zygosity - with more than a third of twin pairs being misclassified in some series (see Scarr & Carter-Saltzman 1979). Comparison of recognized MZ twins (MZ and reared as “identical”) with unrecognized MZ twins (MZ but reared as “nonidentical”) can be used to estimate the role of zygosity stereotyping. On a variety of physical and psychological measures, unrecognized MZ twins seem just as similar as recognized MZ twins (Goodman & Stevenson 1989; Scarr & Carter-Saltzman 1979), suggesting not only that zygosity stereotyping has little impact on these measures, but also that misclassification of MZ twins typically does not result from the two twins differing markedly for nongenetic differences (e.g., because only one twin sustained perinatal damage). Zygosity stereotyping may have more impact on beliefs about twins. For example, although we found that recognized and unrecognized twins were equally similar on objective measures of attention, parent and teacher ratings of hyperactivity were more similar when the twins were believed to be “identical” - a rating bias that, if uncorrected, would almost have doubled our heritability estimates (Goodman & Stevenson 1989). While a comparison of recognized and unrecognized MZ twins provides a measure of zygosity stereotyping, a comparison of unrecognized MZ and same-sex DZ twins provides a measure of true genetic effects uncontamined by zygosity stereotyping. As discussed in greater detail elsewhere, comparisons using recognized and unrecognized DZ twins are less helpful, since either genetic or stereotyping effects could lead unrecognized DZ pairs to be more similar than recognized DZ pairs (Goodman & Stevenson 1989; Scarr & Carter-Saltzman 1979).
To our knowledge, only one small study has used misclassified twins to examine whether genetic or stereotyping effects best account for MZ twins experiencing more similar environments than same-sex DZ twins. Scarr (1968) found that MZ twins were more likely than DZ twins to be dressed alike, and that mothers of MZ twins were more likely than mothers of DZ twins to expect the same levels of social responsibility and independence from both twins. In all these respects, the four pairs of unrecognized MZ twins were treated as similarly as the 19 pairs of unrecognized MZ twins. Though suggestive of genetic rather than stereotyping effects, these findings clearly need to be replicated on larger samples, using a variety of environmental measures that are thought to have an impact on development.
As a first step in that direction, we report here previously unpublished data from a population study of 13-year-old urban twins (described in more detail in Goodman & Stevenson 1989; Graham & Stevenson 1985; Stevenson & Graham 1988). True zygosity was established from biological markers or a twin similarity questionnaire. The sample included 70 recognized MZ pairs, 25 unrecognized MZ pairs, and 111 same-sex DZ pairs. After a lengthy assessment session with parents, a trained interviewer who was blind to true zygosity rated the warmth and criticism of mothers and fathers toward each twin separately, using a modification of the methods used by Rutter and Brown (1966) and Brown and Rutter (1966). In some instances only one parent was assessed (usually the mother). Previous cross-sectional and longitudinal studies of child psychiatric disorder have demonstrated the relevance of these measures of family relationships (e.g., Richman et al. 1982).
Table 1 presents the percentages of twin pairs who experienced the same degree of parental warmth or criticism. It is evident that the likelihood of parents being similarly warm or critical to both twins depends on true zygosity rather than on parental beliefs about zygosity. The figures for recognized and unrecognized MZ twins are closely similar, suggesting little or no zygosity stereotyping. A correlational analysis of this same data indicates that MZ correlations (whether for recognized or unrecognized pairs) are between .07 and .15 higher than DZ correlations, suggesting that genetic differences between children account for about 15-30% of the variance in parental warmth and criticism - somewhat lower than the estimates for family warmth or maternal affection summarized in P&B’s Figure 2.
In conclusion, our data support P&B’s thesis that a child’s genotype can influence aspects of the family environment that affect child development. Though important, this effect should not be overstated. Judging from our data, for example, most of the variance in parental warmth and criticism is unrelated to the nature of the child, that is, the bulk of the variance is environmental in the narrowest sense, being independent of the organism.
Genetic explanations of environment explain little
Philip Graham
In this provocative article, Plomin & Bergeman (P&B) claim that the genetic contribution to environmental influences on child behavior is substantial. The highly significant and important contributions to knowledge made by these authors to our understanding of the categorization and development of temperament should not cloud our judgment in appraising these unexpected and (to many) unwelcome assertions. There are various weaknesses in P&B’s argument. Other commentators will doubtless concentrate on the methodological problems in their interpretation of twin and adoption data. I will consider conceptual issues.
The first conceptual issue lies more in presentation than in content. P&B make a distinction between what they call the “environment” and “environmental measures.” Surely the second should be a simple reflection of the first. The distinction the authors wish to make should be drawn differently. It is between environmental influences that are behavioral (such as the intelligence of the parents and their child-rearing behavior) and those that are nonbehavioral (such as the number of persons per room or atmospheric pollution). The genetic contribution to the former is measurable, while the genetic contribution to the latter is not.
A second concern relates to the quantification of the size of the genetic effect where it exists. Intelligence is a factor in determining socio-economic status, and personality may well be a factor determining life events such as involvement in a traffic accident or an argument with a neighbor. P&B are obviously ambivalent about the level at which they should pitch their claims for the importance of such genetic effects. At one point they say modestly, “Data suggest that nongenetic factors are primarily responsible for variance on environmental measures,” whereas at another point they affirm confidently that “genetic influence is significant and substantial on widely used measures of the environment.” These two statements are not totally inconsistent, but they do convey different messages. The fact is that the data they provide suggest that genetic factors contribute between 10 and 20% of the variance to most of the environmental factors (such as socioeconomic status) that have been examined. It is true that the figure might, in some cases be higher. P&B are right, for example, to point out that their methods of assessing the degree to which MZ-DZ differences elicit different parental behaviors underestimate the genetic influence because they fail to take into account traits directly inherited by the parents as well as, incidentally, the degree to which genetic factors influence the parents’ behavior toward each other. The size of these genetic influences in unknown and is probably small, however. As others will probably point out, the methodological problems of assessing small genetic effects using these methods with any degree of precision are considerable.
Finally, there is the issue of the practical significance of these findings. P&B have no need to make claims for the practical value of their work; their contribution to knowledge can stand as highly significant without the claim that discovering environmental influences free of genetic causation could indicate those that are more likely to be responsive to Intervention. But, as they have made this suggestion, it needs appraisal. Data P&B themselves present suggest that uncontrollable life events are less genetically determined than controllable life events. Are uncontrollable really more likely than controllable life events to be amenable to intervention? Are P&B really suggesting that the fact that the correlation for television viewing is 0.48 for nonadoptive siblings and 0.26 for adoptive siblings should discourage us from interventions designed to encourage children to spend their time less passively? I think the practical significance of this work lies more in the indication it provides that there are indeed genetically determined limits to the degree to which equalization of opportunity will result in equalization of outcome, but it should be comforting to parents, teachers, and policy makers to know that these limits appear to be so wide that they have little relevance to their activities.
“Significant and substantial” or minor and unreliable genetic influences on measures of the environment?
David A. Hay
There is no doubt that Plomin & Bergeman (P&B) have raised an important issue. The possibility needs to be more fully explored that there may be genetic variation both in reports of one’s perceived environment and in those measures of the environment such as maternal interaction, which involve people rather than just objects. There are great implications for our conceptualisation of the “environment,” if more thorough analyses show this to be the case.
I have two major concerns, however. First, the conclusion in the last paragraph “that genetic influence is significant and substantial” summarises an emphasis throughout the text on the magnitude of genetic effects that is not supported by the data. My second point is that P&B may have got their models wrong and that there may indeed be a role for shared environment in contrast to what is indicated here and in other papers (Plomin et al. 1989). The implication of both these concerns is that an approach focusing only on the genetic component may divert us from the major determinants of scores on these measures.
My first concern is obvious from most of the tables. Genetic estimates are small and virtually never significant at better than the 5% significance level. Then in Table 7 we have a footnote indicating that while the genetic estimates based on these correlations are not significant, those reported elsewhere based on the corresponding mean squares are. Such results do not speak of a robust phenomenon.
Added to this is the fact that in all cases there are multiple measures of aspects of the environment and in each Table only a few of these reach significance. P&B try to convince us that there is no risk of a Type I error in that the results are consistent in terms of which aspects of the environment show genetic variation. From the work of Rowe (1981; 1983) and of Loehlin and Nichols (1976) they argue that genetics matters more for aspects of parental warmth than control. To do this they have to convince us that spanking is “more a matter of warmth than control,” which seems more a matter of post-hoc rationalisation than anything else! Then in contradiction of their claim, both on their Sibling Inventory of Differential Environment (Table 6) and on videotape measures of mother-child interaction (Table 8), there is as much significant genetic variation for measures of control as for measures of affection. P&B downplay the importance of the significant control measures in Table 8, but in fact it is the largest difference between non-adoptive and adoptive siblings in the Table.
I appreciate that these are difficult measures to work with, but this low power of analysis becomes a major issue when P&B try to integrate the diverse findings in Figure 2. There is simply no relation between the videotape data in Table 8 and the claim in Figure 2 that these measures have a heritability close to 50%.
I feel that P&B have tried to go too far too fast. This new area of behaviour genetics needs to begin with three fundamental Issues:
(1) The reliability of the measures. The old adage about “heritability never exceeding reliability” is particularly relevant here and applies obviously to the very retrospective nature of much of the reporting. It is important not to confuse the “subjective-objective” dimension In Figure 2 with reliability. The three 5-minute videos of mother-child interaction in the Colorado Adoption Project may be very objective, but they are not necessarily reliable.
(2) The dimensionality of environmental reporting. Exploratory factor analysis will undoubtedly reveal multiple dimensions of any of the environmental measures discussed in this target article, but that does not imply that all these factors are important. Analysing each separately will increase specificity, but possibly at the cost of robustness compared with global measures derived from the whole scale. In Table 7, therefore, the only consistent genetic component on the Home Observation for Measurement of the Environment is for the General Factor. (P&B do refer to genetic variation on the Toys and possibly on the Restriction-Punishment dimensions, but nothing is significant.) As Martin et al. (1989) indicate, behaviour genetics now has powerful techniques based on confirmatory factor analysis to determine the underlying genetic and environmental structure of multiple measures. My feeling would be that these “environmental” measures may show a particularly high general factor because of the retrospective nature of much of the data and the way in which different aspects of the questions contaminate each other: If you are thinking back on your childhood, will parental warmth and control really be rated independently of each other?
(3) The question of sample size. Given such modest heritabilities, just how large would samples have to be to give reliable estimates, especially when, as in Figure 2, P&B are trying to compare the relative magnitudes of such estimates? The simulations in Martin et al. (1978) suggest that few existing studies would be anywhere close to large enough.
My second point concerns the adequacy of the models P&B have fitted to the data. In Table 1 (adult twins’ ratings of their current family environment) the MZA correlation is larger than the MZT on three of the four scales that show significant heritabilities and sometimes quite considerably so. For example, for Expressiveness the correlations are .37 and .22, respectively, and for Control, .36 and .18. in Table 11 (adult twins’ ratings of life events) this pattern is even more marked and the MZA correlations are higher than the MZT on each of the five scales.
None of the conventional genetic and environmental models P&B have tested take this issue into account. One possible explanation is that MZ twins reared together strive to find ways to indicate their individuality or at least to minimise the opportunities for comparison between them. As a result, the variable In Table 11 where the MZA correlation has the most marked difference from the MZT is Controllable Life Events. This is the one in which the MZT can do the most to actively differentiate from each other. A practical example of what this means comes from a recent study of vocational choice in the adolescents in the LaTrobe Twin Study (Whowell & Hay, in preparation). Many twins spoke of how the other twin had chosen the same career or tertiary course they had wanted and that they therefore had to find an alternative. Rosambeau (1987) gives many anecdotal reports of similar phenomena, including the press for parents to be seen to treat their MZ twins differently.
The implications of this possibility are twofold. First, more thought must be given to a realistic parameterisation of situations in which this phenomenon may apply. For example, it is less likely for the measures in Table 9, where the adult twins had to rate the social support they received and the environment is provided by other adults and does not arise directly from themselves or their parents. The more detailed accounts of Plomin’s model-fitting to the data of Table 1 (e.g., Plomin et al. 1989) indicate some problems trying to fit models incorporating shared family environment and genetic dominance (which have the same expectations in both MZA and MZT). Relaxing the constraint that the shared family environment must be the same for all four types of twin (MZA, MZT, DZA, and DZT) may help resolve this issue.
The second point is that this phenomenon would lead to an underestimation of any genetic component, especially in the majority of studies where only twins reared together are available and not those reared apart. Critics of the twin method, such as Kamin (1974), always emphasise how the unrepresentative nature of twin data may lead to an overestimation of heritability, but here the reverse would apply, because the MZ correlation is being lowered relative to the DZ.
P&B’s target article is an important first step toward what may be a major new area of behavior genetics, but the current evidence for genetic variation is at best modest and the measures and the interrelationships among their scales need to be resolved. More thought needs to be given to how these measures may need different models from those applicable to the conventional phenotypes such as personality, now that people are being asked not to describe themselves, but rather how others act toward them.
By making such a strong statement about genetics and about the lack of shared family environment, P&B have gone beyond the existing data in ways that may have hindered the development of this concept. They conclude with many ideas about how this area could be explored further in behaviour genetics, but do not address the fundamental question: Can a study of the necessary magnitude be warranted in our existing state of knowledge?
There is indeed no substitute for multivariate genetic and environmental analyses
John K. Hewitt
Plomin & Bergeman (P&B) are to be commended for drawing the attention of psychologists and social scientists to what is common knowledge among behavioral geneticists, namely, that in the absence of randomized experiments there is nothing we can measure about an individual (including “environmental indices”) that can be a priori assigned to the domain of the purely environmental or social or, indeed, genetic. In each case, the extent of the genetic variance associated with the measure is an empirical question as is, perhaps more importantly, the genetic and environmental covariation between measures. For this reason, P&B’s discussion of multivariate genetic analyses including environmental indices and behavioral measures is, if anything, insufficiently prescriptive. Anything less than the full multivariate genetic analysis, for example, “regressing out” the influence of an environmental index and analyzing the residuals, can mislead us in our attempts to understand the nature of variation in the dependent variable, either because the “environmental” index is in fact partly genetic, or because the implicit assumption about the causal direction is false; or, finally, because the regression of the dependent measure on variation in the index depends on the provenance of that variation (Hosteller et al. 1990).
The first point is the subject of P&B’s target article, the second is alluded to throughout. The third point is a little more subtle but can be illustrated by noting that a phenotypic correlation of zero could arise in principle from a positive environmental covariance together with a negative genetic covariance between two variables. A multivariate genetic analysis would resolve this, whereas quite clearly the traditional “phenotypic” treatment of the covariate would be misleading. In practice the genetic and environmental components of covariance will differ less dramatically.
It comes as no surprise, therefore, to find that measures labelled “social” are not necessarily socially determined and that measures labelled “environmental” do, at least in part, reflect genetic variation. What is now needed is for preconceived ideas to be set aside so that: (1) genetically informative data (e.g., from MZ and DZ twins) can be collected routinely in epidemiological surveys; and (2) appropriate multivariate genetic analyses can be carried out to determine the actual situation rather than that presupposed regarding the variation and covariation of the measured indices. As a practical matter, researchers interested in honing their analytical skills in this area could do a lot worse than attending one of the Methodology Workshops organized by Eaves and his colleagues (see, e.g., Heath et al. 1989; Martin et al. 1989) and sponsored last year by NIMH.
Obfuscation of interaction
Jerry Hirsch
Congratulations to Peter and Roberta Schonemann, whose commentary in this issue clarifies the technical confusions and reveals to us all “where the errors went” in this misconceived and uninformative target article: The second part of the title - genetic influences on environmental measures - has been reiterated no less that 64 times in Plomin & Bergeman’s (P&B’s) short text. Very recently, in an attempt to justify continuing the heritability estimation enterprise, Plomin has assured and then reassured BBS readers that “the message from the literature is that it is difficult to demonstrate G x E [interaction]. . . . [I]t is a lot easier to talk about G x E than it is to find it” (Plomin 1990, p. 144). Wahlsten (1990, pp. 146-55) has shown this to be a fallacy.
I urge BBS readers to heed the advice in this journal of commentator Oscar Kempthorne, universally recognized as an expert in quantitative genetic analysis: “Most of the literature on heritability in species that cannot be experimentally manipulated, for example, in mating, should be ignored” (Kempthorne 1990, p. 139).
As I have previously pointed out:
Heritability estimates cannot be made for human intelligence measurements, because the heritability coefficient is undefined in the presence of either correlation or interaction between genotype and environment, both of which occur for human intelligence. When correlation exists, either (1) between genetic and environmental contributions to trait expression, or (2) between environmental contributions to trait expression in both members of a parent-child or sib pair, heritability is not defined. . . . None of the statements about proportional contributions of heredity and environment to the determination of level of “intelligence” or of many other human traits can be either substantiated or disproven by any conceivable observations. . . . [A] proper understanding of heredity, ontogeny, and statistical-genetical analysis leaves no doubt that general and quantitative statements about “intelligence” and about proportional contributions to it are beyond the purview of science. (Hirsch 1981, pp. 33-34; many pertinent references are given here)
To supplement this comment and further appreciate what is at issue, see Hirsch (1990) and Wahlsten (1990).
In an apposite discussion that deserves to be widely read, Roubertoux and Capron (1990) explain that an intelligence test score is a composite of subtest scores, where different subtests are responsive to different influences. Capron and Duyme (in press) have shown “the differential sensitivities of the Verbal and Performance scales [of the IQ test] to the postnatal environment. The observed effects, according to the scale and the SES of the biological parents, present opposing amplitudes. The absence of interaction on the global IQ is the result of the sum of these two effects which cancel out each other.” Roubertoux and Capron go on to show:
The full IQ which is obtained by adding the two effects cannot under these conditions reflect an interaction. . . . [T]his raises the question of the relevance of a composite measurement in the testing of interaction effects. Failure to find an interaction in this case is a statistical consequence that stems from the composite nature of the test, and does not imply that the factors act additively on IQ.
Roubertoux & Capron’s closing comment as well as their epigraph are relevant here. “Is it so useful to perform a genetic analysis with an IQ score, a meaningless sum? We can perform a genetic analysis with the size of tomatoes, the weight of potatoes, the color of lettuce, but is it reasonable to perform genetic analysis of soup . . . ? This is nevertheless what human behavior geneticists do for IQ.”
I close with their epigraph from Shakespeare: “Much ado about nothing.”
Genes and environment: A complicated affair
Ronald C. Johnson
Much of what is said herein has been said before, but it deserves saying again. Possible environmental influences on claimed-to-be genetic data (e.g., the question of how differences in environmental similarity between MZ and DZ twin pairs might influence MZ/DZ resemblances) have long been assessed and debated, but the reverse - genetic influences on supposed environmental measures - have almost always been ignored. For example, parental socioeconomic status has been regarded as a key predictor of offspring educational/occupational attainment (as in the widely quoted Youth in Transition publications, Bachman 1970; Bachman et al. 1978), even though parental SES is associated with parental ability (as well as with parental personality), both under some degree of genetic control As Plomin and Daniels (1987) demonstrated, across family (e.g., SES) lines environmental variance is far less important than is commonly believed and actually consists of genetic as well as environmental variance, as shown by Plomin & Bergeman (P&B).
Snyderman and Rothman (1990) demonstrate that discussions of genes and environment in the mass media are couched in terms of heredity versus environment and nearly always strongly favor the environmental position. They also show that social scientists and educational specialists have moved beyond the heredity versus environment position and believe that both genes and environments substantially influence individual differences in scores on ability and aptitude tests. The outdated heredity versus environment position is not dead, however. There really are two parts to P&B’s message: (1) heredity and environment are important influences on individual differences, and (2) genetic and environmental influences are intimately related but their influences can be distinguished from one another, given the proper (rarely used) research strategies. This message, if conveyed often enough, may influence future research designs.
Let me list a few specific comments and complaints concerning the target article. (1) One might expect that what were originally small differences between siblings would, through differential treatment, become greater over time, resulting in greater differences in treatment with increasing age. The mother-child interaction data in Table 8 show stability in correlations across age groups for nonadoptive sibling pairs, but a sharp decrease between ages 2 and 3 for adoptive pairs. The CAP subjects are now a good deal older; did the differential trends in treatment similarities continue? (2) Across the various studies reported, the correlations, even when significant, typically account for only 10-15% of the variance, even with less than perfectly reliable tests, this leaves a lot of the variance unaccounted for; P&B might have concerned themselves with this matter. Something important is going on out there that neither behavior geneticists nor persons specifically involved in environmental assessment are measuring. (3) I suspect that the closest parallel to G in intelligence that can be found in the realm of social behavior is liberalism-conservatism. Martin and others (e.g., Martin et al. 1986) have shown liberalism-conservatism to have a very substantial genetic basis. Here is a dimension of individual differences that is under substantial genetic control and might be expected to result in marked differences in the environment provided by parents as well as in one’s self-selected environment. An exploration of this domain might be a better example of genetic influences on “environmental” measures than those provided in P&B’s report.
Minor points notwithstanding, this is an important paper. What is said here has been said before, but P&B say it well. What they say needs to be said again, because only repetition will lead to a shift toward research strategies that will assess more accurately the relative influence of biological and environmental variables on human variability.
A psychiatric perspective on the “nature of nurture”
Kenneth S. Kendler
Plomin & Bergeman’s (P&B’s) is a stimulating article that will serve its goal of encouraging others in the behavioral sciences to think about and investigate genetic control of environmental variables. In the spirit of encouraging further thought and discussion of this important topic, I would like to raise the following points.
First, although it is a catchy phrase, the title, “The nature of nurture,” is actually somewhat misleading. P&B do not sufficiently emphasize the division in classic quantitative genetics of environmental variation into “shared” and “individual specific” forms. Their Figure 1 shows only a single kind of environment represented by the symbol E, usually used to refer to individual specific environment. Their title, however, implies that they are looking more at genetic influences on shared environment or “nurture.” Genetic influences on life events, which are typically regarded as individual-specific variables, is not an examination of the “nature of nurture,” but rather, the nature of individual specific environmental experiences.
Second, P&B take the position that “environments have no DNA and can show no genetic influence.” They take great pains, therefore, to point out that their discussion focuses on measures of the environment, rather than on the environment itself. Their view is a narrow one, however, and it is not shared by some evolutionary biologists. As persuasively argued by Dawkins (1982), it is neither logical nor defensible to consider the skin as the outer limit of gene expression. Genetic variation in several species of birds directly influences nest construction. In such species, can we really argue that body shape (within the skin) is a “real” phenotype, but nest shape (outside the skin) is not? Dawkins goes on to show that such “extended phenotypes” are particularly common in inter- and intra-species interactions. The high-pitched scream of a lost rodent pup is selected for, and has its major phenotypic effect, on the central nervous system of the mother. When we sneeze with a cold, we may be expressing the cold virus’s genotype that has been selected as a way of assisting in its transfer to new hosts. I find Dawkins’s position more realistic and stimulating than that of P&B. In man, whose behavioral repertoire is so complex and interacts with the physical and social environments to such a degree, it is, from an evolutionary perspective, hardly tenable to suggest that gene action stops at the skin. Human environments can be a direct reflection of DNA just as more classic “within skin” phenotypes are.
Third, I want to comment briefly on the implications of this target article for my major area of interest: the genetics of psychiatric disorders. Traditionally, psychiatric genetics has been content to demonstrate that a given disorder is influenced by genetic factors. More recently, efforts have been made to apply quantitative genetic models to psychiatric symptoms and traits in an attempt not only to detect genetic influences but to estimate their size, as well as the role of shared versus specific environmental factors. To a clinician, this static view of the impact of gene action on risk for psychiatric disorders is not very realistic. Most psychiatric disorders probably arise from a complex interplay between individuals and their physical and social environments. As illustrated by P&B’s article, to grasp more realistically the complexity of the syndromes they study, psychiatric geneticists will need to examine a broader range of gene actions than hitherto considered. In particular, genes may alter risk for psychiatric disorders by influencing pathogenic or protective features of the environment.
As reported by P&B, preliminary data from a large population-based twin study conducted here at the Medical College of Virginia also yielded heritable components for certain categories of life events as well as certain measures of social support. It is plausible that a major pathway through which genetic factors influence the risk for psychiatric disorders is one in which individuals create for themselves low risk environments (few life events and high social support) versus high risk environments (lots of life events and low social support). Almost certainly, the genes influence temperamental variables, which in turn influence the predisposition to life events or social support. Impulsive, irritable individuals will tend to precipitate life events. Some individuals are, by temperament, simply more lovable than others.
For all who are interested in understanding the relationship between genetics and human behavior, the points made by Plomin & Bergeman deserve serious thought. Although human genetics in general and psychiatric genetics in particular are moving toward reductionist molecular models, it is vital to counterbalance this effort with the kind of theorizing and empirical work exemplified by their article. As we move downward to the DNA to characterize the genetic variation responsible for human behavioral traits, it is important also to move out into the environment to understand the inevitably complex interplay between our genes and the environment around us.
Different parental practices - Different sources of influence
Hugh Lytton
In a way it is surprising that it should still need stating that measures of parental behavior are partly genetically influenced. But the Plomin & Bergeman (P&B) target article serves a useful purpose in facing us with the broad range of what may be considered genetic influence in ostensible measures of the environment; it thereby helps readers reconceptualize these issues and think about their ramifications. I keep having to clarify for myself precisely whose genes can be said to influence which measures in which studies: (1) Some research shows that measures of parental behavior are an expression of the parents’ own genotype, for example, the Swedish Adoption Study, cited by P&B. (2) Other studies, on the other hand, show that parental behavior is influenced by, and reacts to, the child’s genotype, for example, Rowe’s studies (Rowe 1981; 1983) and to some extent my own (Lytton 1980).
P&B note an interesting difference among parental rearing practices, namely, that a substantial genetic influence (based on offspring genotype) has been detected for parental acceptance-rejection, but very little for parental control (e.g., Rowe 1983); they ask why this should be so. Perhaps I may shed some light on these findings by relating them to some of my own, which concerned the direction of the influence between parents and children (Lytton 1980).
Combining Rowe’s findings and mine, we may speculate that the reason acceptance-rejection is influenced by the genetic characteristics of the offspring (Rowe 1983), is that in the area of attachment, closely related to acceptance/rejection, influence runs mainly from the child to the parent; that is, it is the child’s attachment behavior that determines consequent parental behavior - which may include acceptance - much more than the other way round (Lytton 1980). [See also Lamb: “Useful Distinctions in Human Sociobiology” 10(1) 1987.] On the other hand, the fact that control behavior is not influenced by genetic characteristics of the offspring (Rowe 1983) may perhaps be explained by the finding that in the area of control-compliance, influence runs much more from parents to child (Lytton 1980). This implies that control behavior is more influenced by parental predispositions, which may in turn be partly genetically controlled, something that Rowe’s findings do not preclude. We may argue speculatively that in the area of attachment, and also acceptance/rejection, the child is the dominant force, because parents do not set out on a purposeful program to create or direct attachment to them, and acceptance is also largely a matter of the child’s disposition and relationship with the parent, rather than the parent’s conscious choice. On the other hand, most parents do engage in a conscious, purpose-driven program of shaping and controlling the child’s behavior - hence it is their dispositions that count here. [1]
My own study of 2-year-old twins did not attempt to analyze parental behavior by behavior-genetic methods. In a follow-up of these twins, at about age 9 (cf. Lytton et al. 1986; 1988), the twins rated their parents’ attitudes and practices (e.g., punishment, warmth, restrictiveness, reasoning, amount of interaction, etc.). Intraclass correlations of these ratings between MZ twin partners were often higher than for DZ twin partners, but the difference between them was significant only for the mother’s amount of interaction and play, that is, only this characteristic seemed to be influenced by genetic predispositions of the offspring. The small sample size and measurement errors in ratings by 9-year-olds may explain the absence of genetic determination.
In seeking the origins of human characteristics the problem is that all measures of influences on the child’s development - other than biochemical influences, such as lead in the atmosphere or perhaps historical events - are amalgams of organismic-biological and environmental forces that are difficult to tease apart. In general, influences on the child can be thought of as spanning a spectrum, from biological at one end to environmental at the other, with most influences showing various degrees of admixture in between. In research that relates both parental treatment of the child and the child’s existing or earlier characteristics to child outcome, it is useful to distinguish between “parental” and “child effects.” In such research parental effects are generally a working approximation to effects from the environment and child effects, a working approximation to effects from the genotype, as well as from the biological environment (the womb) and the early social environment (cf. Lytton 1990).
But, as P&B argue convincingly, some designs can also demonstrate the existence of genetic influences of various kinds on such “parental effects,” for example, in the case of biological parents in adoption studies. Path analysis of models of environmental and genetic sources of influence in various family constellations, for example, biological and adoptive families, take a step toward disentangling and quantifying environmental and genetic influences (cf. Coon et al. 1990). But such a demonstration depends on the acceptance of the logic of the path-analytic model and on assumptions some of which may have been made for reasons of expedience in order to identify a given model.
Behavior-genetic methods still essentially depend on a comparison of different black boxes from the outside, with the researcher making a variety of assumptions (in the guise of “models”) about their contents. Often a number of different models are equally defensible for (“fit”) a given data set and all provide plausible quantitative parameters, even when the assumptions of genetic structure change from model to model. Hence a decision between them will be based on personal theoretical predilections or notions of “parsimony.” Plomin & Bergeman are no doubt basically right, but many questions about genetic influence on this or that behavioral domain will be settled more definitely with the expected advances of molecular-genetic research into the human genome.
NOTE
1. Although attachment measures in my study of 2-year-olds did not show any significant genetic loading despite the relationships reported above, a slight genetic influence was discernible in a follow-up at age 9 (Lytton et al. 1988).
On genes, environment, and experience
Matt McGue, Thomas J. Bouchard, Jr., David T. Lykken, and Deborah Finkel
Plomin & Bergeman (P&B) are to be congratulated for their thoughtful review of research on the heritability of environmental measures. Their work identifies a serious shortcoming in much behavioral science research, namely, the misattribution of environmental influence. It is no more defensible to designate a measure a priori as environmental than to designate it as genetic. Recognition of this fact requires a reevaluation of much psychosocial research. Psychological theories on the influence of “schizophrenogenic” and “refrigerator” (in autism) mothers have been disconfirmed by behavioral genetic research (Gottesman & Shields 1982). We suspect that, when evaluated within a broad biosocial perspective, genetic factors will also be implicated in many other supposed environmental influences. Just as it was never permissible to investigate genetic associations without control for environmental factors, so it is improper to investigate the role of the environment without considering possible genetic influences and gene-environment interactions.
Research in this area is only at the initial stages of inquiry; it is largely empirical and there is a need for theoretical models and replication. Our own work challenges some of P&B’s empirical conclusions while supporting others. In a study of personality similarity among twins reared apart (Bouchard & McGue 1990), we found only modest heritability (average of .21) for the 10 primary scales of the Family Environment Scale (FES). We speculated that the modest heritabilities could be due largely to retrospective bias, the personality similarity of MZ twins resulting in similar biased recall of their rearing circumstances. This seems especially likely when respondents are asked to recall ambiguous circumstances such as whether or not their parents were warm or their family close-knit. We recently completed a study of the reliability of retrospective reports of rearing circumstances that appears to confirm this hypothesis (Finkel & McGue, submitted). Individuals high as compared to low on neuroticism retrospectively rated their rearing environments as lower on nurturance, yet the parents of these individuals did not do so, either originally when the individual still resided in the home or retrospectively at follow-up. Although cognitions concerning parental treatment might directly influence psychological status, this did not appear to be the case here, as retrospective reports of parental nurturance did not predict neuroticism at the original assessment period, that is, at the time the parents were or were not allegedly nurturant.
On a more positive note, we have replicated Plomin et al.’s (1990) findings on the heritability of life events. In a recent dissertation at the University of Minnesota, Moster (1990) analyzed the life event reports of reared-apart twins, finding that events that could have been controlled by the individual were substantially more heritable (heritability, h2 = .51 ± .13) than those that appeared to be largely outside the individual’s sphere of influence (h2 = .18 ± .13). The inheritance of life events appeared to be mediated primarily by the individual’s psychological status, as one twin’s level of depression significantly predicted the cotwin’s life event score in MZ but not DZ twin pairs.
P&B’s results require the development of a terminology to describe the nature of environmental influence. In 1909, Wilhelm Johannsen proposed the term phenotype to distinguish what is inherited (the genotype) from what is expressed once modified by the environment (the phenotype). P&B seem to propose an analogous dichotomy when distinguishing the environment from the “measured environment.” Although the conceptual point is important, we suspect that most readers will find this terminology somewhat cumbersome, as P&B apparently do. It is not only, as suggested by P&B, the process of measurement that injects characteristics of the individual into the FES or life event surveys. Rather, genetic characteristics of individuals influence the nature of their peer group, the nurturance of their parents, and the stability of their marriages.
The argument is better made by distinguishing the physical, biological, and psychological conditions of existence (i.e., the organism’s environment) from those aspects participated in, attended to, or encountered (i.e., the organism’s experiences). Experience reflects aspects of both the environment and the experiencing person (our Figure 1). It is experience, not the objective environment per se, that affects behavior, determining what Cattell (1982) calls the “threptic” component of the phenotypic variance. A television set is a shared feature of many environments. How that television set is experienced, however, is a complex function of each individual’s interests, personality, attitudes, and intelligence. We disagree with P&B’s contention that the terminology of genotype-environment correlation is too narrow for what they are talking about. Correlations between characteristics of individuals and their environments have been termed passive genotype-environment correlations; the influence of the individual on its experience (including cognitions) have been termed evocative and active genotype-environment correlations (Plomin et al. 1977; Scarr & McCartney 1983). In any case, we agree with P&B that an individual’s experienced environment is, like IQ, extraversion, and religious attitudes, a phenotype. How much of the variance in such phenotypes is associated with environmental and genetic factors is an empirical question that can only be addressed through the use of behavioral genetic designs.
All these findings make it crucial that we distinguish the distal and proximal determinants of behavior (Rushton 1988). Behavioral geneticists now regularly emphasize that features of the environment mediate the relationship between the inherited genotype and the behavioral phenotype. Bouchard et al. (1990) have argued that “the proximal cause of most psychological variance probably involves learning through experience, just as radical environmentalist have always believed. The effective experiences, however, to an important extent are self-selected and that selection is guided by the steady pressure of the genome (a more distal cause)” (p. 227). For example, the distal cause of all behavioral sex differences is genetic (i.e., the inheritance of an X or Y chromosome). The proximal causes, however, might include sex-specific socialization practices (i.e., an evocative gene-environment correlation) or sex differences in leisure-time interests that translate into different sets of experience (i.e., active genotype-environment correlations). Heritability coefficients estimate only the proportion of phenotypic variance associated with genetic differences among individuals. Although these statistics draw attention toward certain etiological processes and away from others, they do not, by themselves, identify the causal mechanisms underlying trait variation.
We agree with Plomin & Bergeman that we need a deeper understanding of the “nature of nurture.” The next step in achieving this understanding will be the development of measurement instruments and observational methods sensitive to the individual’s role in constructing experience. “Off-the-shelf measures like the FES, although they serve behavioral geneticists well in this first generation of studies, will be of relatively little use in the future. Ultimately, our interest is in determining the extent to which experience influences human behavior. Other than to behavioral scientists, the observation that individuals somehow affect the nature of their experiences will hardly come as a major revelation. The alcoholic engages in self-destructive behavior, the sociopath surrounds himself with like-tempered peers, and the extrovert seeks social stimulation. The real challenge to future behavioral, genetic research is to move beyond our naive biometric models and try to understand how, through genotype-environment correlations and interactions, similar environments are differentially experienced. In doing so, we may well find that the minor role accorded environmental factors in our heritability calculations conceals a wealth of experiential effects.
Three shocks to socialization research
David C. Rowe
In traditional socialization research, one child in each family was studied and parental actions were assumed to mold this child’s personality development. Traditional socialization research has suffered three shocks. The first came in 1968, when Richard Bell proposed that the direction of causality in many socialization correlations had been misread, with children’s personality influencing their parents’ childrearing tactics, rather than vice versa. The second shock came from several decades of behavioral genetic research. It was the absence of “shared” environmental influences on personality development (Plomin & Daniels 1987; Rowe 1990; Rowe & Plomin 1981). The third shock is that our environmental measures are themselves contaminated by genetic influence. Plomin & Bergeman’s (P&B’s) conclusion is not surprising when one realizes that (1) “environmental” measures are usually measures of someone’s behavior, and (2) human behavioral traits are pervasively influenced by heredity.
Each shock weakens the claim that parental behaviors influence personality development in children; each challenges assumptions about what is important for children to become effective adults possessing particular traits. The “causality” shock indicated that the total family-environment/child-behavior correlation may have overstated parental influence. The “shared environment” shock may dash hopes for “main effects” of family environments. If children exposed to similar rearing conditions are not alike, then what power do those conditions have to mold personality? If “main effects” are nonexistent, then what improvement can we anticipate for social interventions recommending that parents adopt different childrearing tactics?
The last shock renders most socialization research useless because it has been done on biologically related family members. If genes can passively create correlations between our measures of family environment and our measures of child behavior, how can we trust the correlations as guides to causal processes? Meta-analysis is useless here. The cumulation of results from a flawed research design cannot lead to a reliable conclusion about family socialization effects.
We probably do not yet see the full range of implications of these results. I believe that process models of socialization must be changed to accommodate them. There are affective and informational explanations for the socialization process. The affective explanation is that children will be permanently affected by emotions induced in them (e.g., distress and fear versus security and love). If this is true, then the rearing dimension of acceptance-rejection should have strong effects on children. We find the expected correlation, but it does not appear to be causal. The implication is that the induction of emotions produces temporary effects, but development is canalized such that the induction of negative emotions fails to leave permanent scars, and that of positive emotions, permanent protection (Clarke & Clarke 1976). Evolutionary theory suggests that there is canalization of development against the impact of predictable stressors; this may be what we observe.
Modeling is an informational theory of socialization effects. Accordingly, children who observe a rewarded parent may adopt their behaviors. This view of behavioral transmission, however, neglects two further possibilities. First, children will sample from many models other than the parents. In the area of sex role transmission, children appear to be influenced by the behavior of a majority of same-sex models, and they imitate a model whose behavior typifies that of their sex but not one who did not exhibit such behavior (Bussey & Bandura 1984; Perry & Bussey 1979). Sampling multiple models avoids the mistake of modeling parents who are deviant on a behavioral dimension. Second, behaviors are tested before they are adopted (e.g., a weakling makes a poor bully). Behaviors inconsistent with genetically induced traits may be abandoned even if they work for others. The canalization of development, the generality of learning, and the testing of behavior suggest that long term effects of parental behavior on child development may be very weak.
If these ideas do not discourage a search for family effects, perhaps the most promising place to look is the dimension of parenting with the least genetic content: family control. Family control may show little genetic content because parents feel compelled to treat children as evenhandedly as possible and because rules are set down for all children. Correlations between the control dimension and child outcomes may not be genetically mediated. On the other hand, the affection dimension of child rearing appears to pull in more correlates with child behavior than any other dimension.
The target article focused entirely on individual differences. The finding that environmental measures contain genetic variability, however, raises issues about cultural differences, as well. The mean genotype of any group would be merely the average of its individuals. The childrearing style adopted by a particular group is usually treated as an environmental variable, independent and separate from the genotypes of group members. If the measure of environment is also partly a measure of genotype, however, then group differences in environment may be a result of group differences in mean genotypes, rather than cultural history or tradition. In the study of cultural differences, interpretations of the relations between rearing styles and child outcomes are clouded.
In conclusion, we have solid evidence of genetic influence on measures of “environment” - naming a measure “environmental” does not make it so. Both Rowe and Plomin (1981) and Plomin and Daniels (1987) suggested looking for environmental influences within families, that is, environmental influences that operate to make siblings different from one another. If the differential treatment of children is a result of reverse causality, however, as suggested by the observational data in the target article (with greater treatment differences for more genetically dissimilar siblings), then parenting style might not be a major within-family environmental influence. Another approach may be to identify specific personality/treatment interactions. Finally, given what we have learned, I believe that theories of socialization process must be changed to accommodate the decreased evidence for long term family environmental effects on personality development.
Origins of nurture: It is not just effects on measures and it is not just effects of nature
Michael Rutter
Plomin & Bergeman (P&B) provide a convincing argument for the importance of considering genetic influences on “environmental” measures and of the value of treating environmental measures as phenotypes. They described the position that they are adopting as “radical,” but it is not radical enough. To begin with, the key question concerns genetic influences on the distribution of environments, and not just on environmental measures. The fact that environments have no DNA is irrelevant; the issue is not whether genes cause particular environments, but rather whether they play a role in influencing population variance in the distribution of environments as experienced. That would be the case even if these were “pure,” entirely objective, error-free indices of the environment.
P&B argue that the evidence for genetic influences on environmental measures challenges the assumption that measures labelled as environment are in fact measures of the environment. That is a logical non sequitur. The processes involved in the determination of individual differences in the experience of environments and the processes involved in the effect of such environments on individual behavior may be the same or entirely different. It is possible, therefore, for an environmental feature to be entirely genetically determined but, for the effects of that environmental feature nevertheless to be entirely environmentally mediated. For example, the single gene disorder of Huntington’s disease is associated with an increased likelihood of family discord (not unexpectedly, because it is a disease with terrible consequences). The family environment of discord in turn seems to predispose to conduct disorder through environmentally mediated mechanisms (Folstein et al. 1983). In this instance, family discord operates as an environmental effect, although that environment has been brought about in large part through genetic influences. The situation whereby the origins of an environmental feature and its effects on the individual reflect different mechanisms is probably quite a common one. Another example is provided by smoking and lung cancer. The mechanisms involved in determining why some individuals smoke and others do not are entirely different from those that mediate the carcinogenic effects of smoking.
P&B do indicate that it is important to investigate the various contrasting mechanisms possibly underlying genetic effects on the environment, but this point needs greater emphasis than P&B have given it. The finding that there is a genetic effect on the environment is not in itself very useful unless one knows something about the processes it reflects. It may reflect a direct effect on the phenotype that creates the environment. Parents who are highly intelligent not only pass on genes influencing intelligence but also, as a result of their own high intelligence, act in ways that influence the upbringing of their children. A related mechanism concerns the ways in which people shape their own environments. For example, numerous follow-up studies from Robins’s (1966) classical follow-up of children attending a child guidance clinic onwards are consistent in showing that individuals who show conduct disorder in childhood have a much increased rate of adverse experiences in adult life (such as unemployment, marital breakup, and lack of social support). Another alternative is that people may select environments even though they don’t shape them. For example, families choose which schools their children attend and that choice has substantial effects on their children’s educational progress (Mortimore et al 1988; Rutter et al. 1979; Smith & Tomlinson 1989). A further alternative is that the characteristics of an individual may influence how environments impinge. For example, we found that in homes characterized by family discord, hostility tended to focus on children with a difficult temperament (Rutter 1978).
P&B focus on genetic influences on the environment. That is a reasonable approach, but it is important to recognize that it constitutes only one subset of a much broader question, namely, how individual differences in environmental experiences arise. Curiously, this is a topic that has received scarcely any attention in the literature on psychosocial risk factors. Yet, it has to be a critical question. Some individuals experience many, and some only a few, acute and chronic adverse life experiences. It is obvious that such experiences are not randomly distributed in the population, so one needs to ask how and why individual differences in experiences arise. One explanation is that genetic factors play a part, it is important to appreciate, however, that an individual’s own behavior may have important effects on the environments encountered even though that behavior may be environmentally rather than genetically, determined. For example, it seems that genetic contributions to antisocial behavior in childhood are relatively modest, but, as noted, antisocial individuals act in ways that serve to create environmental adversities and stressors for themselves. Also, it is often the case that one type of adverse environment predisposes to others. That was evident, for example, in our follow-up of institutionally reared children (Quinton & Rutter 1988). A still different explanation is that the distribution of adverse environments is shaped by societal forces such as ethnic discrimination or housing policies. The investigation of genetic effects on the distribution of environments is one important research endeavor, but genetic factors constitute only one source of environmental variation that needs to be considered.
In summary, Plomin & Bergeman are to be congratulated both on raising an important issue and on presenting a well-reasoned case for the probable importance of genetic influences on environmental measures. Their arguments must take Into account the following, however: (1) Genetic effects may influence the distribution of environments (and not just of environmental measures). (2) Finding that an environmental feature is entirely genetically determined does not necessarily mean that the effects of that feature are not entirely environmentally mediated. (3) The finding of genetic effects on the environment needs to be a starting point for investigating possible contrasting processes that may mediate that effect (rather than a conclusion in its own right). Finally, (4) the investigation of the effects of nature on nurture is just one subset of the much broader and very important question of what factors influence individual differences in environmental experiences.
The construction of family reality
Sandra Scarr
Developmental psychologists have begun to adjust to the idea that behavior is not entirely caused by externally imposed, environmental events. They have even come to recognize that some of the resemblances between parents and children may not arise only out of the ways parents rear their children. It is recognized that children affect their parents’ treatment of them and that, at least for intelligence, there is a considerable heritability in the United States and other Western populations that causes children’s scores to be correlated with those of their parents.
There seems to be less acceptance of parent-child transmission of genetic variance in personality and other behavioral characteristics. The idea that social attitudes, such as authoritarianism, religiosity, and conservatism are, in part, genetically transmitted from parents to children, and not just learned at the parent’s knee, is hard for many developmentallsts to swallow. Despite numerous publications on these findings, they are not yet prominent in developmental textbooks (the litmus test of acceptance in the field).
For Robert Plomin and others of us who Investigate genetic variation in behavior, the struggle has been to get our colleagues to pay more than “lip service” to genetic transmission of behavior in their own research on families. For the past 10 years, we have advised that studies include more than one child per family, so that within-family, nonshared environmental differences can be studied. We have advised that more twins and biological and adoptive siblings be included in ordinary developmental studies, so that genetic variance can be taken into account In interpreting results. There has been only slight progress on that front.
Now we show developmental psychologists that their favorite measures of the environment are heavily contaminated with genetic variance. Will the bad news never cease?
Why the resistance? Why the resistance to the idea that parents transmit genes to their children, with the consequence that their children resemble them, to a modest extent? Because behavioral scientists understand genetic transmission to mean that nothing can be done to change the unfortunate lot of people who inherit bad genes. And this is correct In that so far nothing can be done to affect mild mental retardation (so-called “cultural-familial retardation”), conduct disorders, psychopathy, or any other complex behavioral liabilities, nor is any cure in sight. The futuristic promise of gene replacement therapy may apply by the turn of the century to single gene disorders, but it will not be applicable to multigenetic behavioral characteristics. But environmental interventions have not proved very useful in Interrupting the development of psychopathological disorders, either. One reason is that the environment is in many ways a product of the person and the person’s perception.
There is no evidence that family environments, except the worst, have any significant effects on the development of familial retardation, conduct disorders, psychopathy, or other common behavioral disorders. The behavior genetic evidence, as Plomin and Daniels (1987) stated it, is that differences among families’ environments have little to do with differences in children’s behavioral outcomes. Furthermore, the evidence that die-hard environmentalists cite In support of their hope that family environments do have some influence on behavioral outcomes is challenged by this new evidence that self-reports and ratings of family environments have systematic genetic variance. Favorite family assessment Instruments, such as the HOME and the FES, are shown to reflect both the partially inherited characteristics of the participants In rated environments and the partially inherited cognitive perspectives of the viewers.
Two principles of the environment. This leads me to suggest two principles and some corollaries:
(1) The environment is best construed as an array of opportunities for behaviors to develop and occur.
(a) In the absence of opportunities, development cannot occur. (b) In the presence of opportunities, development may or may not occur, depending primarily on the inherited characteristics of the person.
(2) Environments have nonlinear effects on behavioral development.
(a) Environmental qualities are distributed as a threshold characteristic, with a steep slope across low values and a flat slope across adequate to superior ranges. (b) There is a steep slope of behavioral development across the range of low quality environments because poor environments restrict people’s opportunities to develop their potential characteristics. In this range, improvements in opportunities have large payoffs in improved development, as demonstrated by early intervention programs with very disadvantaged children (e.g., Ramey et al. 1985). (c) There is a flat slope of behavioral development across environmental qualities in the adequate to superior range because adequate opportunities are sufficient to permit genetic variability in the ability of people to take advantage of them to become the dominant source of individual differences. There is little evidence that superior environments, in socioeconomic terms, enhance children’s development above those of average environments (Scarr & Weinberg 1978; 1983).
Kathleen McCartney and I (Scarr & McCartney 1983) proposed that in most respects people make their own environments, primarily through the reactions they evoke in other people and the choices they make from the array of environmental opportunities. Obviously, if people do not have opportunities to learn music, geometry, or cooking, they cannot develop those skills. If they do not have opportunities to play with peers, have parents, or be on a sports team, they will not be able to develop related characteristics, no matter what their genetic complement. Bad environments are restrictive ones; this includes neglectful and abusive homes.
Given that the vast majority of children in Western societies have wide arrays of environmental opportunities, it stands to reason (supported by research evidence) that individual differences in who develops what depend more on the person’s characteristics than on differences in opportunities to learn. Public schools, libraries, sports clubs, and the like are great levelers of family disparities in opportunities afforded children. Egalitarian provisions raise the heritability of personal and intellectual characteristics in Western populations.
Family environments are also diverse opportunity structures for members, each of whom brings a set of personal dispositions to act in, react to, and construe the family environment. The personality match between parents and child, the personal characteristics of the child and parents all affect how they interact in and perceive the family environment. Can it be surprising that the family environment is then a product of the actor and perceiver? Philosophical constructivists have been telling us this for some time.
Plomin & Bergeman have done developmental psychology a great service by showing that the most widely used instruments of “family environment” are not simply environmental measures. They have advanced a view that developmental psychology must accommodate. The theory that, to a great extent, people make their own environments may not be popular, but it accounts for these data and other developmental phenomena.
We wondered where the errors went
Peter H. Schonemann and Roberta D. Schonemann
Because this is already the second BBS target article on twin research in fewer than four years, it attests to the undiminished popularity of Plomin and Bergeman’s (P&B’s) topic, and also to its imperviousness to valid criticisms that were raised the first time around.
In his commentary on Plomin’s previous target article (Plomin & Daniels 1987), Scott (1987) focused attention on the discrepancies between excessive heritability claims by psychologists and the much more modest findings of animal researchers, who derive their estimates from controlled mating experiments. King (1981, p. 82) reports heritabilities for milk production in cattle of the order of 30%. Yet psychologists boasted “heritabilities of IQ” in the 80% plus range ever since Galton (1869), until Henderson (1982, p. 411) informed us recently that now “an estimate of 50% seems more in vogue.” This is still much more impressive than the paltry 25% for staple length of wool in sheep (King 1981). Loehlin and Nichols (1976) reported a mass of MZ/DZ intraclass correlations that transform into stunning “heritabilities” even for the most unlikely candidates: For item 250, “Had your back rubbed,” the correlations imply narrow heritabilities of 92% for males and 21% for females, incidentally raising the question of why backrubbing is more heritable in males.
Scott has advanced an elegant hypothesis to explain these persistent discrepancies between animal and human heritabilities: Could it be “that the genetic model commonly used is inadequate, being based on an oversimple set of assumptions about the nature of gene action and interaction” (Scott 1987, p. 40)? As Bock and Zimowski (1987) observed, anyone wishing to check this would need an explicit statement of the model from which the estimates were derived. But the present target article meticulously avoids any explicit statement of the model and refers the reader instead to two previous publications for the “SATSA model employed in these and subsequent SATSA reports.” On checking them (Plomin, McClearn et al. 1988; Plomin, Pedersen et al. 1988), readers will find that the models are not the same in the two papers, leaving them in suspense as to which model P&B had in mind: Plomin, McClearn et al. use “the standard biometrical model (e.g., Jinks & Fulker, 1970) [which] fits a model of three parameters: heredity, shared environment, and nonshared environment. In addition, we added a parameter to represent selective placement” (p. 740). Plomin, Pedersen et al., on the other hand, introduce a “nonadditive genetic parameter” in the latter part of their paper (p. 45), whereas the bulk of the paper uses the simple additive component model (in our notation):
y = a + e + z,
where a is an (additive) genetic variable, e an environmental variable, z is measurement error, and the variables on the right are uncorrelated (see Appendix details). This “standard biometrical model,” which involves neither placement nor nonadditive genetic effects, is the model that underlies the correlations on p. 45 in Plomin, Pedersen et al. (1988).
We wondered where the measurement errors went that normally afflict personality tests, and discovered that they pose as “nonshared environment” in this model, which has implications for narrow heritability estimates. As shown in the Appendix, under this simple model the reliabilities (systematic over total variance) are estimated either by the intraclass correlations of the MZTs or by the sum of the correlations for the DZAs and DZTs. The MZA correlations, on the other hand, estimate var(a)/[var(a)+var(e)+var(z)], which is narrow heritability confounded with measurement error (not “narrow heritability,” as P&B claim in sect. 2.1.1). Since this parameter changes whenever the length of the test is changed, it is useless. Instead, the model implies that the ratio rMZA/rMZT and Nichols’s (1965) HR (:=2(rMZT-rDZT)/rMZT) both estimate narrow heritability corrected for unreliability, (var(a)/[var(a)+var(e)]). The fact that these estimates frequently exceed unity simply means that the “standard biometrical model” rarely fits the data.
Of course, Scott is right that a basic caveat governing all “modelling” is that the whole model may be wrong, in which case one may wish to entertain a qualitatively different model. Before putting any faith in parameter estimates, including “heritabilities,” therefore, one should first ask (a) what the model is, (b) what testable assumptions it implies, and (c) whether the data violate them.
We found such systematic violations in the celebrated Shields (1962) data on MZs, which completely invalidate Jinks & Fulker’s (1970) bold conclusions. We also found that a purely environmental model that postulates correlated environments for MZAs not only eliminates most of the negative variance estimates Jinks and Fulker obtained, but also fits quantitatively better by a factor of 2 (Schonemann 1989; 1990). Similarly, on reanalyzing Osborne’s (1980) MZ/DZ personality data, Schonemann and Schonemann (1988) found that the MZs were much too similar compared to the DZs than the “standard biometrical model” allows. As a result, most narrow heritability estimates HR exceeded unity and many variance estimates were negative. After fitting a purely environmental model with the same number of parameters, all variance estimates became nonnegative and the quantitative fit improves by a factor of 14.
In view of our past experiences, we could not resist the temptation to apply similar tests to P&B’s data. As shown in the Appendix, two easily checked consequences of the model are (a) that the correlations are ordered rDZA ≤ rMZA ≤ rMZT ≤ 2rDZT and (b) that Nichols’s HR estimates narrow heritability cleansed of measurement error. The results of these checks are in our Table 1. We permuted the four leftmost columns of Tables 1, 5, 10, and 11 from the target article, so that deviations from the predicted order are more readily seen. As Table 1 shows, both reliability estimates are usually close and rather low, as one might expect for short self-report tests. Only the data of Table 5 (second block of our Table 1) have respectable reliabilities. On checking the correlations, one finds that most data of Tables 1, 10, and 11 violate the order implied by the “standard biometrical model.” In particular, the MZTs are more similar relative to DZTs than the model predicts, just as we found was the case for Osborne’s data in (Schonemann & Schonemann 1988). One also finds that many Nichols’s ratios HR exceed unity, just as they did for Osborne’s personality data in Schonemann & Schonemann (1988). Only for the data in Table 5 are all HRs admissible.
Since we found that most of P&B’s data violate the defining assumptions of the “standard biometrical model,” we suspect that a purely environmental model of the type we proposed in Schonemann & Schonemann (1988) might fit them better, with the possible exception of those in Table 5 of the target article. Such a comparison between qualitatively different models requires the within and between mean squares, however, which P&B, unfortunately, did not provide.
APPENDIX
The “Standard Biometrical Model” and some of its implications: This model explains each observed score as a simple sum of three random variables (“random effects”): an additive genetic effect a, and environmental effect e, and a measurement error z:
y = a + e + z.
On using subscripts when the effects differ for the two twins (e.g., ev e2 for twins raised apart) and omitting them if both twins share the same effect (e. g., e for twins raised together), the model describes the observed deviation scores yk (k = 1, 2) for the various twin configurations as follows:
MZTs: yk = a + e + zk DZTs: yk = ak + e + zk MZAs: yk = a + ek + zk DZAs: yk = ak + ek + zk
where var(a) = var(ak), var(e) = var(ek), var(z1) = var(z2), cor(a1,a2) = .5, all other variables are uncorrelated and the the means are zero.
This model implies the intraclass correlations (see also Plomin, Pedersen et al. 1988, p. 45):
MZTs: [var(a) + var(e)] / var(t) DZTs: [.5 var(a) + var(e)] / var(t) MZAs: var(a) / var(t) DZAs: .5 var(a) / var(t)
(where var(t) = var(a) + var(e) + var(z)), which, therefore, obey
DZA ≤ MZA ≤ MZT ≤ 2DZT.
This predicted order provides a qualitative check on the fit of the “standard biometrical model.”
Inspection of the above four intraclass correlations further shows that the reliability (systematic over total variance) can be estimated either with the correlation of the MZTs or the sum of both DZ correlations, while rMZA estimates var(a)/var(t), that is, “narrow heritability” uncorrected for attenuation. Both Nichols’s ratio, 2(rMZT-rDZT)/rMZT and rMZA/rMZT, estimate narrow heritability corrected for attenuation, var(a)/[var(a)+var(e)]. To be admissible, this estimate must be a nonnegative proper fraction. For more details and extensions to models with dominance deviations, see Schonemann (1989; 1990) and Schonemann & Schonemann (1988).
Environment - A dubious concept?
Fini Schulsinger
Plomin & Bergeman’s (P&B’s) target article revives strong and mixed feelings in the present commentator who is an old-fashioned psychiatric geneticist from the 1960s, when the battle was an “either-or” fight - either environmental or genetic. I was on the genetic side, but recognized the potential role of environment evidenced by the approximately 50% concordance for schizophrenia found in identical twins.
At the time the “nature-nurture” debate in the field of schizophrenia was provoked by Lidz et al. (1965), who failed to note that the “skewed” parental relations of a young schizophrenic patient might very well be an expression of a shared genetic liability within the family. Mednick and McNeil (1968), in a review of methodological pitfalls in schizophrenia research, quoted a number of publications that indicated that parents of children who suffered from severe osteomyelitis, from poliomyelitis demanding treatment in a respirator, or from severe juvenile diabetes showed the same kind of psychological disturbance as Lidz et al. and other groups had found among parents of young schizophrenics. The reviewers used this as evidence to suggest that an environment characterized by severe illness in children provoked measurable psychological deviations in parents. Today, juvenile diabetes and schizophrenia are considered genetically determined diseases, confirming P&B’s concept of a “genetically determined” environment for the parents. But. what about poliomyelitis and osteomyelitis? The victims of these diseases may have a genetically determined weakness - possibly of a specific nature - of their immune defense system.
In other words, the “nature of nurture” opens up, almost as Pandora’s box, a boundless field of possibilities. These may be matched by the growing potential of multivariate statistical methods. This combination of growth, however, needs to be accommodated not only by extremely valid instruments for measuring behavior and social events, but also by very valid hypotheses to test. Otherwise, quantitative behavioral genetics runs the risk of continuously moving around in the periphery of problems.
P&B’s target article is very intelligent and instructive. It describes a new avenue within behavioral genetics characterized by a higher level of complication. Let us imagine that Brown and Harris (1978), before they entered into their pioneering enterprise to find the origins of depression in women, had read a paper like P&B’s. This might have triggered so many speculations about how to separate nature from nurture in the relatives of the depressed women that they might never have started their research. Such a course would have been regrettable, because a lot of useful information came out of the study.
In spite of the increased level of complication Plomin & Bergeman’s paper might provoke, it is an extremely useful reminder to be used in the process of brainstorming during the hypothesis-creating phase of nature-nurture studies.
Genes and genius from Galton to Freud
Dean Keith Simonton
Francis Galton was a pioneer in the early development of what is now called “behavior genetics.” In his first book Hereditary genius (1869), he introduced the family pedigree method; subsequently he showed how twins could help clarify the nature of genetic inheritance (see Galton 1883). He even defined the concepts of correlation and regression that his student, Karl Pearson, elaborated into the methods that have become so central in behavior genetics (Galton 1888). In addition, Galton pioneered the scientific study of genius. His 1869 volume was an attempt to demonstrate that exceptional natural ability, or “genius,” ran in families, thereby marking a clear case of genetic inheritance. Geniuses in all domains of achievement, whether creativity or leadership, were born, not made. When the botanist Alphonse de Candolle (1873) attacked Galton’s position by showing how environmental circumstances affect the emergence of genius, Galton responded in his typically empirical fashion by conducting the systematic inquiry that led to English men of science: Their nature and nurture (1874). Besides introducing the questionnaire technique, Galton coined the terms “nature” and “nurture,” thereby defining the debate that has plagued the behavioral sciences from his day to ours. Galton himself backed away from his extreme nativistic position of 1869 by admitting that specific developmental experiences may nurture the growth of genius.
Although some of Galton’s heirs continued some allegiance to genes as providing the principal origins of genius, the historical trend moved toward nurture as the primary agent. Such a shift was probably encouraged by the advent of American behaviorism, which, at least at the start, adopted an extreme environmentalist stance, as observed in the classic debate between William McDougall and John B. Watson in 1924. This emphasis on nurture was certainly conspicuous in the literature that emerged concerning creativity, gifted children, and other topics related to the study of genius. Researchers seemed set on discovering just the right combination of child-rearing techniques that would guarantee every conscientious parent a genius child. Educational systems, too, were closely scrutinized for what they might contribute to the nurturance of prodigal intellects. For the past three decades or so, the prevailing Zeitgeist seems to endorse the view that genius is more made than born. This viewpoint has the asset of being optimistic, besides enjoying more compatibility with an egalitarian culture in which all citizens are born equal, and would continue equal if granted identical developmental opportunities.
Yet one implication of Plomin & Bergeman’s (P&B’s) target article is that this shift from nature to nurture may have gone too far. At least some of the “environmental” factors taken as influencing the appearance of genius may actually represent measures of underlying genetic dispositions. P&B already suggest one possible example: The cultural, intellectual, and aesthetic enrichment that seem so conducive to the development of future achievers may in truth serve as proxy indicators of an outstanding native intellect, and not just that of the parents. One of the lessons to learn from Feldman’s (1986) studies of child prodigies is that many parents, when confronted with an astonishingly precocious child, reconstruct their lives and the home environment to meet the child’s ever expanding needs. This practice has a long history, as can be witnessed from reading the lives of such eminently gifted children as Pascal or Freud, but not all supposed developmental factors can be relegated to this subordinate position. On the personal level, the impact of a child’s birth order, which has a robust even if weak association with distinction, cannot be dismissed as a mere repercussion of genetic givens (cf. Simonton 1987). And at the societal level, a respectable number of sociocultural and political conditions have been linked to the personal development of the eminent (Simonton 1984). These external inputs operate on far too large a systemic plane to be susceptible to deflection by an individual’s puny batch of DNA. Thus, we cannot expect the pendulum to swing entirely back to the position that Galton adopted in 1869. His more moderate compromise of 1874 between nature and nurture is the one most likely to survive scientifically intact once the P&B thesis is allowed to work its way through subsequent research. Still, current inventories of developmental influences probably understate the importance of good genes for genius.
Freud was mentioned in passing a moment ago, and I would like to return to him. Notwithstanding his deep roots in physiology and medicine, Freud, as far as genius is concerned, looked to nurture rather than nature. This orientation can be seen in his development of psychobiography, such as his classic work on Leonardo da Vinci (Freud 1964). The whole point of these retrospective psychoanalyses is to discern the early developmental experiences responsible for distinctive aspects of a luminary’s personality. In line with classic psychoanalytic theory, the pertinent etiological factors are to be found in early childhood and almost invariably involve parent-child relationships. Yet if the P&B thesis is taken seriously, some if not all of these hypothesized environmental influences may in fact represent parental responses to a child with a characteristic genetic constitution. It may be, for example, that a child with a native affinity toward things rather than people - a future natural scientist at heart - will neither encourage nor expect close affective bonds with parents, and the parents will respond accordingly (cf. Roe 1953). When this possible inversion of developmental causality is considered, it would seem that the psychobiographer’s task must be far more complicated. Before childhood experiences are evaluated as environmental causes, they must first be appraised as potential genetic effects, an assessment that may require the detailed scrutiny of family pedigrees a la Galton. And if epistasis is at all conspicuous in the genetic endowment of genius, the obstacles to an exhaustive genetic analysis may become insurmountable.
Hence, if the central position of the target article has any scientific merit, the study of genius, whether from the nomothetic perspective of Galton or from the idiographic viewpoint of Freud, must be radically transformed. Only with trepidation can we allot to nurture what might more justly belong to nature.
Problems with the “environment as phenotype” hypothesis
Radomir Socha
Despite several decades of criticism of the view that the behavioural phenotype can be partitioned into inherited and acquired components, the dichotomous nature/nurture approach remains wide-spread and influential. Plomin & Bergeman (P&B) attempt to breathe new life into this traditional, oversimplified nature/nurture opposition by introducing a new “nature of nurture” dimension. According to P&B, it is not only the behavioral phenotype but also the environment treated as phenotype that can be decomposed into genetic and environmental determinants. My commentary will deal more with its conceptual than its methodological aspects. Below I illustrate some conceptual complications inherent in this gene-centered, deterministic approach to the environment.
Nobody doubts genetic influences on the environment if they are measured and analyzed as a phenotype of the individual. The analyses used in this research program are rather complicated, however, and the results obtained not informative enough and very problematic, especially because of the extreme distance of the final measured characteristics from the initial gene action. The remoteness lies in the number and complexity of intervening steps (Bonner 1987), interactions and transactions between various levels and components (Hofer 1981; Johnston 1988), organism specificity (Wachs & Gruen 1982), hierarchical self-organizing properties of the biosystems (Thelen et al. 1987), hierarchical organizational constraints on development (Maynard Smith & Vida 1990), feedback between nongenetic and genetic components (Socha & Zemek 1978) and many other factors. Taking the above into consideration, the informational content and interpretation of P&B’s results turn out not to be so straightforward as they believe.
It is known that the genes that participate in the organism’s’ ability to “transmit” its individual experience to offspring by learning - so that the organism is acting on and constructing its own environment and not just passively reacting to it - can influence parent-offspring relations and even the evolutionary trend of a given species. We must not forget, however, that there is a deep difference between talking about genes “determining” certain behavioral patterns and behavioral environments and genes taking part in the developmental construction of these patterns. Transgenerational continuity or “fixation” of newly originated behavioral or sociocultural patterns that drive the organism into a new environment or allow it to construct its own new specific behavioral environment need not be genetically predetermined. The stability of such a transgenerational pattern can occur even without its rigid genetic fixation, that is, without being encoded in nucleic acids of germ cells (Socha & Zemek 1982). The repeated generation or “fixation” of various transgenerational changes (so-called generons) in sociocultural patterns - for example, the transmission of new song patterns in birds (Jenkins 1977), or of human native languages - in the sequence of generations can be ensured by nongenetic mechanisms (Socha 1990a; 1990b). There exist various organizational levels and informational channels in biosystems and their surrounding biosphere where various kinds of generons can be repeatedly generated or transgenerationally fixed (“memorized”; Socha & Zemek 1990). The production of hereditary (transgenerational) characters cannot be reduced to a purely genetic affair, and characters repeatedly generated in the flow of generations nongenetically cannot be treated as nonhereditary. Such an understanding of hereditary characters accords well with a broader multilevel concept of heredity (Zemek et al. 1985) in which “hereditary” information is not reduced to genetic information. In spite of the fact that some generons are not genetically fixed, they can be classified as hereditary and can acquire an important role in parent-offspring relations or even in the evolutionary processes. A hierarchical-systemic model of heredity respects the autonomy and specificity of various hierarchical organizational, informational, and evolutionary levels, and at the same time goes beyond the orthodox gene-centered deterministic and transmissional concept of heredity and evolution.
P&B’s genocentrically oriented research on the “nature of nurture” phenomenon is rather problematic if one considers that there exists no rigid genetic predetermination of the way organisms must interact with their environment. It is well known, even for insects, that certain kinds of responses to the same environmental signals are not prescribed in a genetic information program beforehand but are rather contextual, and their construction depends both on the sequence of environmental signals and on the developmental history of the organism, that is, the sequence of its interactions with environments (Socha, in preparation; Tomioka & Chiba 1989). Some behavioral patterns are accordingly more stable than others and are the preferred outcomes in particular contexts yet not rigidly predetermined. This is in agreement with Gyama’s view (1985) that “ontogenetic information,” whether about the body or behavior, does not exist in the genes or in the environment, but is constructed in a given developmental context. The concrete ontogenetic or behavioral pattern is not preformatively encoded in the primordial genome, but results from epigenesis of the whole organism and sometimes also that of the genome. No blueprint of the final behavioral pattern is encoded in genes (Socha 1990a; 1990b). I am convinced that the same can be said about behavioral environments, in particular, the environment conceptualized by P&B as the phenotype.
Finally, I would like to mention the possibility of an analogous line of thinking, opposite to the one underlying P&B’s radical concept of the environment as phenotype. It is also quite possible to think about “environmental influences on genetic measures.” New findings have shown the genome to be more flexible than it was formerly thought to be; it can change during ontogeny (Cullis 1975; McClintock 1951; 1978). Neither mutability nor genetic linkage represent a physical constant, but they are truly biological, that is, functionally controlled processes with many entrances for signals from the changing internal and external environments of the organism. Various types of genomodulation (e.g., imprinting, rearrangements, structural alterations, etc.), even the transgenerational ones, may occur as a result of the action of various factors coming from various levels of the internal and external environment (Cullis 1975; Pollard 1987; Socha & Zemek 1990). It has been shown recently that even adequate or adaptively directed mutations can be induced during an organism’s response to specific environmental stress (Cairns et al. 1988).
In the future we may also take into account the influence of the scientific knowledge “environment” on genetical measures. By using genetic engineering methods we may be able to induce goal-directed changes in various genes and thereby change developmental pathways and evolutionary trends in various plants, animals, and other organisms, including man. I see a new “Lamarckian” kind of human evolution fast approaching that could stimulate future research on the “nurture of nature,” provided that this unfruitful, dichotomous nature/nurture concept is still alive in this progressive scientific era.
Modeling and measuring environment
Auke Tellegen
Plomin & Bergeman (P&B) present convincing evidence that widely used scales purportedly measuring psychologically significant environmental variables often have a substantial genetic component. The evaluation of environmental measures is important, since the larger aim of understanding the environment itself cannot be realized without it. This aim requires maintaining a clear distinction, stressed by P&B, between “the environment” and “measures of the environment.” My view of this distinction differs from theirs, however, and may provide a clearer perspective on the implications of their thought-provoking analysis.
P&B propose to treat environmental measures as phenotypes. If we define “phenotype” traditionally, as the genetically and environmentally determined observable characteristics of an organism (which presumably include its behavioral characteristics), then P&B’s proposal is reasonable and helpful. It encourages efforts to characterize empirically rather than a priori the attributes tapped by environmental measures.
P&B’s own a priori judgment, however, is that the environment “itself cannot be considered a phenotype, and, more specifically, that it can show no genetic influence, on the grounds that “environments have no DNA.” This view leads them to conclude that the evidence for genetic influence on environmental measures “challenges the reasonable assumption that measures labelled as environment are in fact measures of the environment.” Yet earlier on they reject the position that “finding genetic influence on environmental measures means that the measures are not really measures of the environment,” pointing out that “then by definition there can be no genetic influence on environmental measures,” presumably not a tenable position. The apparent contradiction suggests ambivalence. My own view is that P&B’s position hinders rather than helps a proper appreciation of their analyses.
To settle this issue it may be helpful to ask first what is meant by “the” environment. From a psychological perspective the term refers to the class of potentially distinctive environments of the individual organisms in the population we choose to study. Unquestionably, many important features of these environments vary systematically across individuals. In environmental/genetic models of behavior, these features can be represented hypothetically as latent variables. An environmental latent variable should be distinguished from the (fallible) environmental measures included in the model to estimate the former empirically. The distinction is important; we do not equate “degree of intellectual stimulation,” a latent variable, with “number of books in the home,” a measure. However imperfect our environmental measures, the environmental latent variables we propose are presumably our best current scientific guess as to what the important variations among individual environments are really like.
Researchers undoubtedly tend to view some environmental latent variables as influenced by genetic factors. Their argument would not be that the environment contains DNA, but that the entities containing it have DNA-influenced characteristics, including behavioral characteristics, that tend to place them in certain environments rather than in others. In short, DNA exerts a distal influence. Individual differences in degree of acceptance by one’s peers, degree of exposure to intellectual stimulation or to physical risks, and many other environmental features and their measures are readily thought of as reflecting genetically influenced intellectual and temperamental characteristics, and thus as themselves reflecting genetic variance. Whatever their ideas, investigators are free to incorporate them in their models and test them empirically. If a model fits the data poorly, one has the choice of changing constructs or measures or both, before starting a new round of data collection. Things are no different for genetic/environmental models and measures than they are for models and measures in other domains.
P&B also point out that findings may differ substantially depending on whether we use “objective” or “subjective” (self-report) environmental measures (Figure 2). Subjective and consensual measures of the environment (even if similar in content) might not only reflect different genetic processes, as P&B suggest, but they could plausibly be linked to qualitatively different latent variables representing different “worlds,” one objective-consensual, the other subjective-experiential.
Taking P&B’s lead, I show in my Figure 1 a schemata path diagram illustrating the issues raised: the influence of both genetic and behavioral factors on the environment, and the distinction between measures and latent variables and between the subjective and objective environment. The upper three labeled circles represent genetic (G), consensual-environmental (E), and cognitive-behavioral or psychological (B) latent variables. E and B are linked to designated behavioral and environmental measures or markers (b1, b2, b3) and (e1, e2, e3), respectively, shown in boxes. G is depicted without indicator variables (markers), because behavior-genetic investigators have estimated genetic contributions in the absence of specific genetic markers through the collection of environmental and behavioral data in twin studies, adoption studies, or through other appropriate designs permitting estimates of genetic variance components (and as P&B’s target article shows, until recently E has also been without indicator variables). The circle labeled SE refers to the subjective environment, as explained shortly. The diagram is not technical but expository and depicts an example of model development.
Suppose that B is a Positive-Emotional (“extroverted”) temperamental trait (Tellegen et al. 1988) and that E represents “degree of supportiveness of the individual’s social environment.” Path p then represents the investigators’ hypothesis that Positive Emotionality, as estimated by b1 b2, and b3 is (detectably and substantially) influenced by genetic factors, while path q signifies that persons higher on Positive Emotionality tend to seek out and create more supportive and facilitative social environments. Together, paths p and q portray genetic factors as influencing an individual’s social environment via their influence on personality. Path r (the “nurture of nature” path) represents the hypothesis that a supportive environment in turn tends to enhance one’s Positive Emotionality. Jointly, paths p, q, and r illustrate a “nature via nurture” perspective that we believe to be fundamental to understanding the nature and function of nurture (Bouchard et al., in press). A longitudinal design would be needed for a decisive test of the nature-via-nurture, or G -> B E, hypothesis.
Suppose also that our investigators, having defined E as a consensual feature of the individual’s social environment, then reasonably selected ratings of the individual’s social environment by knowledgeable outside observers as their indicators e2 and e3 but that they optimistically chose a self-report measure for e1. Suppose in addition that persons high on Positive Emotionality (B) tend to form too rosy a picture of their world (as compared to a consensual assessment of their circumstances), causing e1 to be a poor measure of E (while it might still be a reasonably accurate reflection of B). The outcome could be a less-than-satisfactory fit of the current model to the data. This might prompt the investigators to replace e1 belatedly, or to revise their model by, for example, removing path s and replacing it with path t (shown as a thin line in Figure 1), in the hope of achieving a more accurate interpretation of the data and a somewhat improved fit.
One might revise the model more substantially and consider adding a separate “subjective-environment” latent variable, SE (sketched in Figure 1 as an emerging concept), and modeling it as influencing, and influenced by, E and B, or a B-related latent personality mediator, and as estimated by e1, and other now useful subjective measures still to be selected.
Modeling and validly measuring objective and subjective environments may not be so simple. If they are to capture essentials of organism-environment interactions, however, I believe our models will have to represent both these environments and include their major input and output lines of influence, including genetic influence.
Improvisations on the behavioral-genetics theme
Esther Thelen
The assumptions of behavioral genetics are intuitively simple. An individual’s behavior, the phenotype, is determined by two sources of information. The first is what is coded in the genes, inherited from the parents, and “belongs” to the organism in a material and permanent way. The second is all that effects behavior but comes from outside the organism, from the environment. The genes and the environment, and their interactions, comprise a closed set, which, if it could be measured accurately, would completely account for individual variance in behavior. The central question asked by behavioral geneticists is what part of the behavioral phenotype is genetically determined and what part is the result of the environment. They answer this question by ingenious and sophisticated designs involving twins, adoptees, and siblings who share genes and environment to differing degrees.
The issues addressed by behavioral geneticists are among the most fundamental for understanding human behavior and its development. What aspects of behavior are stable over the life span? What are the limits of adaptability and change? The questions are of great importance, I believe, but the basic assumptions of the approach are, at the very least, over-simplified, and probably biologically untenable. Because of the inadequacy of their assumptions, behavioral geneticists, when confronted by their own meticulous data, are compelled to invoke explanatory constructs like “genetic influence on environmental measures,” the subject of the present target article and “nonshared environment” (Plomin & Daniels 1987) to fill in the holes in the model.
The genetic methodology used here by Plomin & Bergeman (P&B) revealed that people affect their environment, and the environment, in turn, affects other people. Stripped of the genetic vocabulary, this is hardly surprising. They have discovered that the partitioning of human behavior into a clean dichotomy, genes and environment, is not so clean after all because the environmental measure is not “pure,” but contaminated by the genes of the people who compose that environment. This sets for the authors the task of partitioning the environment into “pure” environment and “genetic” environment. The people contributing to the “genetic” environment are also presumably contaminated by “environmental” contributions to their genetics, and so on for endless partitioning. (One might also inquire about the variance in the researchers who measure the environment, for even the so-called “completely objective measures such as videotape” are interpreted by people influenced by their genetics and environment.)
This predictment is similar to the one raised by Plomin and Daniels in their 1987 BBS paper so intriguingly titled, “Why Are Children in the Same Family so Different From One Another?” Under the assumptions of behavioral genetics, children in the same families, who share both genes and environment, ought to be more alike than they really are. Plomin and Daniels explained this inconsistency by the construction of nonshared environment, which consists of the systematic, but as yet elusive, environmental differences that affect even children in the same family and engender their unique constitutions. But, we ask, how can anything so hard to measure exert such a whopping influence?
The simple and seductive assumptions of behavioral genetics require these improvisations because they do not do justice to the biological realities of the developmental process, which is complex, nonlinear, and above all, constructive. As many have noted (e.g., Oyama 1985), the genes do not code behavior, but exert their influence through a long, contingent process. During development there are many opportunities for small random variations in organic and environmental conditions to be amplified and to cascade into individual differences that cannot be partitioned into two exclusives sources (Thelen 1989; 1990). These include the nongenetic and epigenetic events of early embryology that determine early morphology and the dynamic, stochastic, and selective nature of neurogenesis (Edelman 1987; 1988). Contemporary dynamic systems theories of development emphasize that the organism and its nuturing environment cannot be logically partitioned because behavior is never context-free; it is always an emergent property within that context. From the earliest days, the organism is constructed in interaction with its surrounds in a manner far more complex and nonlinear than the simple models of behavioral genetics. Plomin & Daniels’s “nonshared environment” reflects, not poorly measured variance, but the very stuff of development: individual differences built by a nonlinear process, and, in principle unable to be “disentangled” into the “cause and effect” (Plomin & Daniels 1987, p. 21) models of behavioral genetics. Likewise, the environment as a phenotype is constructed by whole, intact people with developmental histories, and not by their genes.
A dynamic systems theory of development (DSTD) is a framework to answer the important questions posed by P&B without the genes-environment dichotomy (Thelen 1989; Thelen et al. 1987). DSTD offers an alternative to the linear and additive assumptions of the genetics approach with concepts from modern nonlinear dynamics, especially synergetics (Haken 1983), a theory of pattern formation in complex systems. In DSTD, behavior is an emergent property of the cooperation of the multiple organic subsystems in a particular physical and social context. Because the elements are free to combine in fluid and task-specific ways, causality cannot be assigned to any element in the organism or the environment alone. Behavior is truly constructed during development as certain cooperative interactions of elements coalesce and become stable, while others lose stability and disappear from the behavioral repertoire. Thus, the empirical task is not the statistical analysis of such endpoint traits as IQ, but the tracing of the developmental trajectories of variables that express the cooperation of organism and environment to identify periods of stability and change. A basic assumption is that the cooperation between organism and environment is dynamic, that is, it has a time-history, and is also highly nonlinear. The same behavior may therefore be assembled over time by different routes at the same time that very small differences in routes may be amplified to produce divergent end states. This means that a dynamic analysis is essential to begin to understand which processes and mechanisms are stable within the individual and between individuals, and which are the variables that engender developmental change.
Why do we want to know what part of behavior is “genetic” and what part is “environmental”? If the root question is one of the sources of individual differences (Thompson 1990), the purity of the analysis is already seriously compromised by the so-called genetic influence on environmental measures as well as the fundamental nonlinearity of developing organisms. If the goal is to plan interventions, we want to know about stability and the agents of change. The arbitrary partitioning into genes and environment does not help this task and imposes oversimplified assumptions, which must be strained and modified to fit the data. If understanding developmental process is the aim, P&B are not convincing about what the environment-genes partitioning adds to the conventional longitudinal studies.
Is H2 = 0 a null hypothesis anymore?
Eric Turkheimer and Irving I. Gottesman
It was Robert Plomin (Plomin et al. 1977) who first brought to our attention the following assertion from Roberts (1967): “It matters not one whit whether the effects of the genes are mediated through the external environment or directly through, say, the ribosomes” (p. 218). To which Plomin et al. replied, “In practice it often matters quite a few whits, especially if one should be interested in intervening in the process. Changing behavior by changing parental attitudes is a decidedly different proposition than tinkering with the ribosomes” (p. 321).
It can hardly come as a great surprise that socioeconomic status, ratings of family characteristics, or self-reports of parenting styles have a genetic component. What doesn’t? In recent years, genetic components have been demonstrated for social attitudes (Martin et al. 1986), divorce (Lykken et al. 1990), and indeed for practically every cognitive and personality variable imaginable. Our environment, especially to the extent we can measure it, consists of people, and almost all characteristics of people have turned out to be more or less heritable. When characteristics of people in the environment are measured through their own subjective impressions or those of others, there are two chances to detect genetic contributions, once in the subject and once in the rater.
Our concern is about where all this will lead. Behavior is influenced by genotype and environment. The environment provided by a parent is influenced by the parent’s (not to mention the child’s) genotype, and the parent’s rearing environment, which had its own tangle of reciprocal genetic and environmental influences. Everything is intercorrelated; everything interacts. Where does this leave the columns of “model-fitting heritabilities,” meticulously computed to two decimal places and starred for statistical significance on the basis of path models that cannot hope to keep pace with the reciprocal causal structures described in the target article?
Opponents of the behavior genetic agenda have always argued that the dynamic interaction of genotype and environment was too complicated to permit meaningful decomposition of phenotypic variance into independent genetic and environmental components (e.g., Taylor 1980). Many of these opponents, driven by an ideological aversion to any genetic influence on behavior, proceeded to throw out the genetic baby with the bathwater of variance decomposition. The target article has demonstrated, yet again, the incontrovertible influence of genes on every aspect of human behavior, but in so doing it may weaken our already shaky confidence in the meaningfulness of traditional analyses of heritability - useful as they may have been in the earlier stages of our field. [See also Wahlsten: “Bias and Sampling Error in Sex Difference Research” BBS 11(2) 1988.]
Suppose, unknown to behavioral science, every 10 hours children spent watching Jeopardy added one point to their IQs. What would be the best way to go about discovering and understanding this phenomenon? First, consider the possibility that the tendency to watch Jeopardy may have - undoubtedly has (Plomin et al. 1990) - a genetic component. Children with a higher genetic loading for intelligence may find the show more interesting; intelligent parents may encourage their children to watch “educational” shows; childrens’ inherited temperament may predispose them to watch one kind of show rather than another. Who cares? The important discovery that needs to be made is that an activity exists that can increase IQ, and the important quantification that needs to be accomplished is not to partition the variance of either Jeopardy or IQ, but rather to estimate the magnitude of the effect, that is, one IQ point per 10 hours watched.
The “Who cares?” in the above paragraph is a deliberate overstatement. It may, in some contexts for some populations, be interesting and useful to partition the variance of IQ. But consider that in our example, the heritability of IQ would be a function of the fundamental effect (one point per 10 hours), in addition to the variance of parental IQ (are these children unselected, or all from the same institution, or are they faculty kids?); the variance of Jeopardy-watching (if children do not vary much in number of hours watched, hours watched will account for little of the variance in IQ, and the effect will probably be missed entirely); and the variance of everything else that is influencing IQ. Add to this the myriad complexities stemming from the genetic-environmental cross-influences discussed in the target article, and the second decimals of those columns of heritabilities start to look pretty unimportant.
The point of Plomin et al.’s (1977) profound reply to Roberts (1967) is that variance partitioning can be deceptive, because variance can sometimes be manipulated. It may be that one of the ways nature has engineered for intelligent parents to transmit their intelligence to children is by predisposing such parents to provide facilitative environments, which are then, in some sense, genetic. But the provision of rearing environments is not a fixed aspect of nature: It is to some degree under our control. If social or educational programs enabled the less intelligent parent to provide the kind of rearing environment previously provided only by the genetically advantaged (a big if, itself a matter for empirical investigation), the genetic component of environment would be eliminated.
It is certainly important to remind behavioral scientists that genetic paths need to be included in all models of familial transmission, or for that matter in all models of behavioral development. Nonetheless, the very success of the behavior genetic agenda may have obviated the discipline’s fundamental research paradigm. Having established beyond any reasonable doubt that some of the variance of every human characteristic is influenced by genes, it may be time to enshrine H2 ≠ 0 as the “first law of behavior genetics,” and concede that H2 = 0 is no longer an interesting null hypothesis.
NOTE
1. One might add that “genetic” measures also have environmental components. Biologic parent IQ, a common genetic measure in adoption studies, is clearly a measure of parental genotype and that parent’s rearing environment.
Nature and nurture: A shaky alliance
Theodore D. Wachs
The major theme of Plomin and Bergeman’s (P&B’s) target article is that measures of the environment may contain genetic variance, which can be estimated with traditional quantitative genetic methods. Speaking as an environmental researcher, I have no quarrel with the possibility that genetic factors may be associated with measures of the environment. A paper looking at the interrelation of genetics and environment needs to pay as careful attention to environmental models and measures as it does to genetic models and measures, however. In addition, although the data presented by P&B suggest an association between genetic and environmental measures, it is doubtful whether they can be used to imply genetic influences upon environmental measures.
The need to consider environmental methods and models more closely. A prime example of the need to consider existing environmental models is seen in those sections where P&B discuss the SATSA study. Based on their assumption that the correlation for monozygotic twins who are reared apart “directly estimates heritability,” they conclude that there is a genetic influence on certain dimensions of the Family Environment Scale. Such a conclusion would be valid only if we can assume that being reared in different families means being reared in totally different environments, across all levels of the environment. In making this assumption P&B ignore the contributions of ecological models of environmental action, which emphasize the need to consider multiple levels of the environment simultaneously. For example, as demonstrated by Bronfenbrenner (1986), when we take into account the larger ecological context, phenotypic similarities for separated monozygotic twins may reflect similar ecological contexts as much as similar genotype.
A similar problem is seen in P&B’s treatment of environmental measures. The fact that a measure of the environment has been widely used in the literature does not necessarily guarantee its validity. (An excellent example of this point is seen in a recent review of parental rearing attitude measures by Holden & Edwards 1989.) Whereas it is possible to apply quantitative genetic methods to decompose any measure of the “environment,” from an environmental standpoint it is important to ask what this environmental measure is actually assessing. For example, when discussing data from the Colorado Adoption Project, P&B present evidence on the possible association of genetic factors with “environment,” as measured by brief videotaping of maternal behavior. What is not considered is the possibility that short-term videotaped measures of mothers may be assessing parental reactivity to being videotaped rather than representative parent behaviors (Wachs 1988), or that the environmental component of such brief measures is not likely to be stable across time (Wachs 1987). Given this, it is legitimate to ask whether the data reflect a genetic influence upon environment, parent reactivity or error.
With respect to other “environmental measures” decomposed by P&B, the problems with retrospective reports of the environment have been well documented for more than 20 years (e.g., Yarrow et al. 1970). Similarly, while socioeconomic status (SES) is often used as a measure of the environment, the adequacy of SES as a measure of the psychosocial environment is low, given that SES may also measure genetic variance, variance caused by biomedical factors, or variance resulting from nutritional status. Even with such widely used (objective) measures as the HOME, there are methodological factors that need to be considered - for example, whether the observer is in the home long enough to get an adequate sample of parent behavior, or whether there is sufficient environmental variance to produce meaningful HOME scores in middle class populations.
Again, it must be stressed that there is no quarrel with the general point raised by P&B, that there may be an association between genetic factors and environmental measures. In approaching this problem primarily within a quantitative genetic framework, without detailed consideration of existing environmental models and methodologies, however, P&B have seriously compromised the adequacy of the data presented to support their argument. Dealing with what is essentially an Interdisciplinary question, namely, the interface of genetics and environment, requires more than an attempt to analyze one domain solely from the framework of another domain.
Does correlation equal genetic determinism? There is a very curious dichotomy in this paper. For the most part, P&B approach the nature-nurture question in terms of genotypes “influencing” environmental measures, rather than genotypes being correlated with environmental measures. Yet, the overwhelming majority of the data they present reflects genotype-environment correlation. For example, the SATSA data reported in P&B’s Table 1 is based on twin correlations, and yet P&B conclude that “genetic factors affect the FES.” In discussing the Loehlin and Nichols study, P&B conclude that “heredity affects adolescents’ perceptions of their parents’ treatment,” even though the data reported are correlational (Table 3). Similar deterministic statements about genotype influencing environmental measures are made in regard to the study by Rowe (Table 4) and the Colorado Adoption Project (Table 7), to cite just a few examples. Similarly, Figure 1b and some of the key references cited by P&B basically state that genes drive environments (e.g., Scarr & McCartney 1983) or environmental measures (Figure 1b). Although at certain points in the paper P&B frame their conclusions In terms of gene-environment covariance (e.g., “genetic effects on environmental measures must be due to covariation with genetically Influenced characteristics of the individual”), for the most part, the thrust of their argument concerns genetic determinism rather than genotype-environment correlation.
Acceptance of P&B’s model by environmentalists might be possible in spite of the genetic determinism theme, if it offered the promise of substantial gains in knowledge about how genes and environments jointly contribute to development, which may not be true, however. Consider intelligence: There are many studies showing that at least part of the variance in intelligence is caused by genetic factors. [See Jensen: “The Polythetic Perspective” BBS 11(4) 1988; and “Sex Differences in Arithmetic Computation and Reasoning in Prepubertal Boys and Girls” BBS 11(2) 1988.] The partitioning-of-variance approach has not been particularly useful in telling us much about the processes underlying genetic (or environmental contributions to variability in intellectual development, however. The partitioning-of-variance strategy espoused by P&B may therefore tell us that certain measures of the environment contain some genetic variance, but what will this tell us about the processes wherein variability in environmental (or genetic) measures is associated with variability in development? One approach to studying the process may lie in the use of multivariate genetic analyses, as suggested by P&B. Progress in this domain may be limited, however, by the fact that most multivariate genetic analyses require large sample sizes, which may preclude the use of more accurate and representative measures of the environment, particularly for younger children who are unable to make accurate reports about their perceptions of the environment (Wachs, in press).
In place of a partitioning-of-variance approach there is an alternative model that addresses many of the issues raised by P&B avoids genetic determinism, more accurately reflects their data, and also leads into the question of process. This alternative model is based on genotype-environment correlation, an example of which is shown in Figure 1. In contrast to a partitioning-of-variance approach, a genotype-environment correlation model not only allows us to deal with the central question posed by P&B, namely, the nature of the relation between genotypic and environmental measures, but it also requires us to take the next necessary step, namely, to identify which genetically mediated characteristics of the Individual (either parent or child) are associated with measures of the environment. [See also Wahlsten: “Bias and Sampling Error In Sex Difference Research” BBS 11(2) 1988.]
On the whole, I believe Plomin & Bergeman have done the field a service by suggesting the possibility of an association between genetic factors and measures of the environment. The critical question from an environmental standpoint is whether they are willing to discard what may be an inappropriate theoretical model and analytic method and work more closely with environmentalists to come up with models and methods that do not violate either domain, and yet allow us to make progress on this critical question of determinants of development.
The need for collaboration between behavior geneticists and environmentally oriented investigators in developmental research
Irwin D. Waldman and Richard A. Weinberg
The evolution of the nature-nurture controversy in recent years has reflected the search for an appropriate conjunction to describe the relation between these two influences on human behavior - nature or nurture, nature and nurture, and now, the nature of ‘nurture. The target article is the most recent in a series by Plomin and his colleagues that deals with the estimation of genetic and environmental influences on “environmental” measures and on environment-development correlations. Plomin & Bergeman (P&B) point out that the majority of environmental measures traditionally used In the psychological literature reflect individuals’ behavior and psychological traits either directly or indirectly, and therefore may be subject to behavior genetic analyses like any other phenotypes. The authors marshall a wide-ranging literature that consistently demonstrates genetic, as well as environmental influences on these environmental measures.
In this commentary, we seek to outline briefly four directions for future research dealing with genetic and environmental influences on “environmental” measures and environment-development correlations. We feel that future developmental research would benefit from greater interactions between behavior geneticists and environmentally oriented researchers who could guide the former in the choice of relevant environmental factors to study and of adequate environmental measures.
1. The contribution of active versus reactive aspects of individuals to genetic influences on environmental measures. Genetic influence on environmental measures can arise in a number of different ways. For example, genetically influenced characteristics of parents may influence the rearing environments they provide for their children. Alternatively, differences in rearing environments provided by parents may reflect genetically influenced characteristics of their children. As P&B point out, separating the active and reactive aspects of genetic influences on environmental measures transcends the issue of direction-of-effects discussed in the socialization literature (Bell 1968), as it moves beyond the phenotypic level to consider the genetic and environmental influences underlying socialization processes. Socialization researchers could aid behavior geneticists in the selection of relevant environmental constructs and measures, as well as in determining which of these are least and most related to parental and child characteristics. Nonetheless, creative designs (e.g., Lytton 1977) will be necessary to disentangle active and reactive components of genetic influence on environmental measures.
2. Isolation of method, source, and target factors in genetic and environmental influences on environmental measures. A methodological issue related to the one raised above involves the examination of method (e.g., objective behavioral observations or subjective ratings of behavior), source (e.g., parent or child ratings), and target (e.g., twins as parents or twins as children) factors in genetic and environmental influences on environmental measures. Few studies have examined the effects of more than one method, source, or target. For example, P&B (see also Plomin, DeFries et al. 1988, pp. 272-77) have described the different genetic components of variance in environmental measures that would be isolated by studying twins as children, twins as parents, or siblings in adoptive and non-adoptive families. Clearly needed are multivariate behavior genetic studies that incorporate multiple method, source, and, if possible, target factors. Although it is unrealistic to expect investigators to examine many possible combinations (e.g., both twins as parents and twins as children) in a single study, they may be able to pool results from multiple studies to converge on the effects of these three sets of factors on genetic and environmental influences of environmental measures.
3. Investigation of mediators and moderators of genetic and environmental influences on environmental measures. We agree with P&B that a logical next step is to search for intervening variables that may bridge the chasm between genetic factors and measures of the environment. Bergeman and Plomin (1988) have attempted to identify parental influences that mediate the genetic relation between the HOME and Bay ley IQ scores at 2 years of age. Surprisingly, the authors found that this relation was not mediated by predictable parental variables (e.g., SES, IQ, personality); they suggest that mediating factors for such relations (e.g., “harmony” of family relationships, attributional processes) may lie outside the realm of commonly studied cognitive and personality variables. Another direction for future research would be to search for moderators of genetic and environmental influences on environmental measures. An investigator could examine not only genetic and environmental influences on an environmental measure (e.g., an index of parental responsiveness), but also how these influences may vary as a function of parental characteristics (e.g., IQ, neuroticism). In addition, researchers have criticized both the characterization of the environment (Wachs 1983) and the range of environments studied (Scarr 1987) in behavior genetic research. One could also examine genetic and environmental influences on an environmental measure as they vary across different environments (e.g., different communities, or families of different race, ethnicity, or SES). Such moderating effects could easily be examined using multiple regression analysis of behavior genetic data (see DeFries & Fulker 1985).
4. Developmental aspects of genetic and environmental influences on environmental measures. As P&B suggest, genetic and environmental influences on environmental measures and environment-development correlations may change developmentally. Hence, a logical extension of this research would involve the longitudinal study of environmental measures and outcome variables in behavior genetic designs (see Plomin et al. 1988). Such designs would allow one to examine continuity and change in genetic and environmental influences on environmental measures and environment-development correlations, as well as genetic and environmental influences on change itself in these environmental measures and environment-development correlations.
The above discussion highlights the necessity of incorporating specific environmental measures into developmental behavior genetic research; it suggests fruitful collaborative opportunities for environmentally oriented researchers and behavior geneticists. Environmentally oriented developmental researchers (e.g., Wachs 1983) have complained that the treatment of environmental effects in behavior genetic models is indirect; they have argued for the inclusion of specific environmental measures. For example, in the Colorado Adoption Project, correlations between the HOME and children’s IQ were frequently equal to or greater than parent-offspring correlations for IQ in both nonadoptive and adoptive families (Plomin et al. 1988, Tables 6.4 and 11.4, pp. 151 and 297). In addition, correlations between the HOME and children’s IQ did not appreciably decrease after partialling out parents’ IQ. Hence, incorporating specific environmental measures into behavior genetic models may result in stronger environmental effects on a trait than the estimate of these effects gained by comparing sibling and parent-offspring correlations for the trait in nonadoptive and adoptive families.
Overinterpreting model fitting effects
Lee Willerman
We should thank Plomin & Bergeman (P&B) for documenting that perceived environments often contain variance because of genetic factors. It has been difficult to convince those with strong environmentalist proclivities to give more than lip service to any genetic perspective in their work, and this research might persuade them to do otherwise. Regrettably, sometimes one cannot make much sense of the specific results. Using sophisticated model-fitting programs to compute proportions of heritable and nonheritable variation seems to have run ahead of data quality and theory. Perceived environments as phenotypes may in some cases fail to be psychometrically or biologically meaningful, although numbers can be assigned to responses and heritabilities can be calculated. The model-fitting method further subdivides the variance into additive and nonadditive components (based simply on whether DZ correlations are less than half the MZ correlations), despite no specific biological or evolutionary processes really being inferable. We see in the worst case the use of genetic terminology without much else.
Consider P&B’s Table 11, showing that 40% of the variation in the total life events scale is heritable. The original report (Plomin et al. 1988) revealed that the significant genetic variation in the total score was nonadditive, indicating that it is not transmissible from parent to child, but shared by the identical twins. When the total score was broken down into its components, however, there was significant additive genetic variation (which is predictably transmissible from parent to child) for desirable and uncontrollable events and significant nonadditive genetic variation for undesirable and controllable events, and for self-care (not shown in Table 11). The total score must therefore be composed of additive and nonadditive variation, yet the results suggest only significant nonadditive variation. How can one draw any meaningful biopsychological inferences from such results? Just as the aggregation of the subscales into a total score produces a picture different from the subscales themselves, dividing subscales into subsets of items could well produce a still different picture. One must start with phenotypes that are at least theoretically meaningful and reasonably reliable to have a chance of making any sense of the results to be obtained. Heritability for phenotypes lacking large common factors, for example, will have almost no chance of being explainable.
In the ideal case, the use of separated twins has much to commend it, particularly for measures in which the presence of a cotwin in the home is not likely to affect the results (e.g., IQ). But when a cotwin forms a significant part of the perceived environment, together versus apart comparisons may be misleading because twins reared apart have not had a cotwin in the home and therefore cannot be affected by potential assimilation or contrast rating artifacts. The same phenotypic values may be achieved by multiple and perhaps radically different routes or combinations of items, with the biopsychology underlying the phenotypic value necessarily ambiguous. This is true for even better established measures such as IQ, where different combinations of subtest scores can lead to the same total IQ score, but here at least the fairly substantial general factor running through intelligence scales justifies some fungibility among components.
As P&B note, questions about correspondences of subjective and objective environments remain unanswered. There is good reason to think that recall accuracy could be unacceptably low, however. For example, a follow-up community survey found that more than half the subjects who had reported a depressive episode when interviewed three to four years earlier failed to report ever having had the episode when reinterviewed (Aneshensel et al. 1987).
The median retrospective MZT intraclass correlation of ri = .55 for the abbreviated FES scales in Table 5 can serve as an index of interrater agreement about shared childhood environments. This .55 value suggests the possibility that mood, memory, or other factors are contributing substantially to variance in scale scores while the measure is being completed. One plausible source of variance within pairs is in the reconstruction of past environments based to some degree on current status. Adults who believe that their lives have not gone well might be inclined to have more negative recollections of childhood, for example. To the extent that adult identical twins turn out to be more alike than fraternal twins, recollections of childhood family environment might be more similar. An obvious test of this hypothesis is to partial out resemblance in ratings of current status from ratings of the past and see what remains.
The seemingly dramatic findings about genetic influences on the perceived family environment stem in part from over-inclusive use of the term “environment.” The FES has many items that can be interpreted within an individual personality assessment framework, for example, “We feel it is important to be the best at whatever you do; it’s hard to blow off steam at home without upsetting somebody.” Consequently, subjects’ personalities may enter into the evaluation of their families. Indeed, it should be possible to construct personality scales from some of the FES items that would duplicate and perhaps account for the genetic patterns observed on these “environment” scales. P&B’s results would then not be quite so surprising, comporting with other studies showing personality resemblance for twins reared apart (Bouchard & McGue 1990).
Do the overall results have implications for the adoption situation where there is no genetic overlap between parents and children? Disagreements in social perception can be rich sources of parent-child conflict and may contribute to the increased likelihood of adopted children being brought to the attention of mental health practitioners.
Authors’ Response
Nature and nurture
The point of our target article is very simple. If one takes a measure of the environment and treats it as a phenotype in a quantitative genetic analysis such as a twin or adoption study, one often finds evidence for genetic influence. We intentionally chose to focus on the basic phenomenon – that environmental measures show genetic influence – rather than spinning off into the ramifications of this finding, which we felt would have diluted the impact of the article. In our response to the melange of commentaries, we hope that the forest is not lost for the trees: Nineteen commentaries agree with our general point that the provenance of widely used measures of the environment includes genetics. Two additional commentaries (by Bradley & Caldwell and Graham) seem to accept this conclusion, and for four others it is not clear whether they accept it or not (Duyme & Capron, Hay, Socha, and Wachs). Only five of the commentaries clearly disagree with the finding (Baumrind, Bookstein, Hirsch, Schönemann & Schönemann, and Thelen). The latter commentaries, however, question the use of quantitative genetic methods to investigate genetic influence on any phenotype, not just the application of these methods to the analysis of environmental measures which is the topic of the target article. We begin by responding to the latter commentaries. Next we consider issues concerning the interpretation and implications of the finding. The final section of our Response addresses suggested directions for research on the nature of nurture.
All the new data mentioned in these commentaries confirm the existence of genetic influence on environmental measures. McGue et al. mention dissertation research (Moster 1990) that replicates our finding that controllable life events show greater genetic influence than uncontrollable ones (Plomin, Lichtenstein et al. 1990). Kendler notes that preliminary twin analyses at the Medical College of Virginia also find heritable variance on measures of life events and social support. Bouchard & McGue (1990) replicated our finding of significant genetic influence on the Family Environment Scale (Plomin, McClearn et al. 1988); the average heritability in their study was .21 and in ours it was .25. Goodman & Stevenson present welcome new data concerning the equal-environments assumption of the twin method, specifically in relation to measures of the environment. They find that parents respond more similarly to identical twin children, even when the parents mistakenly think that their children are fraternal twins. We have also become aware of the results of a family study of depression that suggests that a common familial factor predisposes individuals both to depression and to stressful life events (McGuffin et al. 1988).
General criticisms concerning quantitative genetics
As mentioned previously, five of the commentaries question the use of quantitative genetic methods to investigate genetic influence on any phenotype. They raise objections that have been considered for a long time in quantitative genetics. For example, Baumrind is concerned about restriction of range, selective placement, and the unequal environments assumption of the twin method. Schönemann & Schönemann focus on error of measurement and nonadditive genetic variance. (The other three commentaries – by Bookstein, Hirsch, and Thelen – are discussed later.) Such issues have been discussed extensively in the quantitative genetic literature. We had thought it was no longer necessary to provide a general discussion of quantitative genetics in our target article, but these commentaries lead us to begin with a brief statement about quantitative genetics as applied to the study of behavior.
One of the most dramatic shifts in the behavioral sciences occurred during the 1980s, when antipathy toward human behavioral genetics turned into acceptance. For example, a survey of more than 1,000 social and behavioral scientists and educators indicated that most have accepted a significant effect of heredity on IQ scores, traditionally one of the most controversial areas in behavioral genetics (Snyderman & Rothman 1988). [See also Jensen: “‘Total Perceived Value’ as the Basis of Assortive Mating in Humans” BBS 12(3) 1989.] Indeed, with increasing frequency, we caution that the rush of the behavioral sciences away from environmentalism may be going too far, to a view that all behavior is biologically determined. Against this background of remarkable change, it is almost nostalgic for us to see these antigenetic commentaries. For example, diatribes of the sort in Hirsch’s commentary were commonplace 15 years ago; this atavism should serve to remind us how far the social and behavioral sciences have come since the 1970s. Unfortunately, Hirsch devotes his commentary to congratulating people who have written antigenetic tracts rather than addressing our target article, which limits what we can respond to. If behavioral genetics is, as Hirsch says, “much ado about nothing,” why does he need to keep repeating himself as the decades go by, while other behavioral scientists shake themselves free from the shackles of environmentalism and accept a more balanced view that recognizes genetic as well as environmental influences on individual differences in behavioral development?
We have trouble understanding how a serious scientist can any longer deny the ability of quantitative genetic methods to detect genetic influence on complex quantitative traits, including behavioral traits, or deny the results of quantitative genetic research that converge on the conclusion that genetic influences are important. Artificial selection studies provide a powerful demonstration of the impact of heredity on the behavior of nonhuman animals by showing that we can successfully select animals whose offspring reliably and appreciably differ for behavior. For example, in one of the longest mammalian selection studies of behavior, replicated high and low lines were selected for activity in a brightly lit open field, an aversive situation thought to assess emotional reactivity (DeFries et al. 1978). After 30 generations of selection, a 30-fold difference exists between the activity of the high and low lines, and there is no overlap between them.
For human behavior, no quantitative genetic methods as powerful as selection studies exist. Human behavioral genetic research relies on family, adoption, and twin designs. As in family studies of nonhuman animals, family studies of human behavior assess the extent of resemblance for genetically related individuals, although such studies cannot disentangle possible environmental sources of resemblance. That is the point of adoption studies. Genetically related individuals adopted apart give evidence of the extent to which familial resemblance is the result of hereditary resemblance.
Twin studies are like natural experiments in which the resemblance of identical twins, whose genetic identity can be expressed as a genetic relatedness of 1.0, is compared to the resemblance of fraternal twins, first-degree relatives whose coefficient of genetic relatedness is 0.50. If heredity affects a trait, identical twins should be more similar for the trait than fraternal twins. As in studies of nonhuman animals, family, adoption, and twin studies can be used to estimate the magnitude of genetic influence as well as its statistical significance.
Consider a quantitative physical trait such as height. Correlations for first-degree relatives are 0.45, whether reared together or adopted apart. Identical and fraternal twin correlations are 0.90 and 0.45, respectively, again regardless of whether they are reared together or adopted apart. These results indicate significant genetic influence. Heritability is a descriptive statistic of effect size that estimates the proportion of phenotypic variance that can be accounted for by genetic variance. For these height data, heritability is estimated as 90%. These same methods can be used to investigate genetic influence on behavioral characteristics and yield evidence for appreciable and nearly ubiquitous genetic influence (Plomin, DeFries et al. 1990). The point of our target article is that when these methods are applied to measures of the environment treated as phenotypes, they sometimes yield evidence for genetic influence, as well.
Although we feel that the specific points raised by these commentaries are not really the issue, we will respond to them. The treatment of error by Schönemann & Schönemann seems bizarre. They use twin correlations to estimate reliability, but twin correlations can be low when measures are very reliable, which is precisely the case with the data that Schönemann & Schönemann abuse. Similarly, their attempts at a purely environmental model simply ignore the possibility of genetic influence and rename as correlated environments any excess similarity of identical twins as compared to fraternal twins. We groan for the Schönemanns’ contortions as they try to avoid the obvious, parsimonious interpretation of behavioral genetic data: Genetic influence is important.
Concerning Bookstein’s example of “attractivity” between parent and child, how would he explain a finding in which his measure of parent-child attractivity is more similar for identical twins than for fraternal twins, especially for twins reared apart? It is odd that after arguing that there is “no way to claim understanding of genetic influence,” Bookstein concludes that “inasmuch as no one appears to disagree with the target article’s principal claim, one wonders why it was written at all.”
Thelen misconstrues quantitative genetics. Genetic influence does not refer to genes that “belong to the organism in a material and permanent way,” nor does the environment refer to “all that affects behavior from outside the organism.” She attacks as oversimplified and biologically untenable the sketch of a straw man that she has drawn. Thelen also says that because of the inadequacies of their assumptions behavioral geneticists are compelled to invoke constructs like genetic influence on environmental measures and nonshared environment to fill in the holes in the model. This is a strange misrepresentation of what we think are among the most important discoveries that have emerged from human behavioral genetics research. Because quantitative genetic methods make it possible to consider both genetic and environmental influences rather than assuming that one or the other is paramount, quantitative genetic analyses of behavior have led to these two exciting discoveries at the interface of nature and nurture: (1) Environmental influences salient to behavioral development are “nonshared,” that is, they make children in a family different from rather than similar to one another (Plomin & Daniels 1987); and, now, (2) the most widely used measures of the environment show substantial genetic influence when treated as phenotypes In genetic analyses.
In the rest of this section, we consider other issues raised by these and other commentaries that address general issues in quantitative genetics rather than issues specific to the investigation of genetic influence on environmental measures.
Interactionism (Baumrind, Bradley & Caldwell, Bronfenbrenner, Socha, Thelen)
Several commentaries attack quantitative genetics because it does not sufficiently take into account interactions between genetic and environmental effects (Baumrind, Bradley & Caldwell, Bronfenbrenner, Socha, Thelen). In our view, mistaken notions of nature versus nurture have too often been replaced with the equally mistaken view that the effects of heredity and environment cannot be analyzed separately, a view called interactionism (Plomin et al. 1977). Obviously, there can be no behavior without both an organism and an environment. The scientifically useful question is: For a particular behavior, what causes differences among individuals?
Thelen and Socha refer to a “dynamical systems theory of development” presented by Oyama (1985). We agree that behavior is a complex system and that it can involve emergent properties. But to say that genetic and environmental effects interact and therefore cannot be disentangled is wrong. It should be noted that Oyama accepts the validity of quantitative genetic research. For example, she states that she is not “disputing the possibility or utility of genetic analysis or of accounting for phenotypic differences by specific environmental or genetical variation” (p. 3; see also, pp. 37-38 and 86). We agree with exhortations that we should be “unpacking the developmental system to determine temporal priority, level of analysis, transfer of control, and interrelatedness of variables” (Oyama 1985, p. 165). These exhortations would have more impact, however, if they were accompanied by a plan for research to accomplish these worthy goals.
The empirical findings that have emerged from quantitative genetic research on human behavior are novel and exciting, and are emerging at an accelerating rate. This pace will quicken as quantitative genetics begins to capitalize on the spectacular advances in molecular genetic techniques, developments that presage a bright future for this growing field. We are not aware of similar advances made by interactionists – proponents of the “dynamical systems theory of development (DSTD)” – who seem to spend more of their time telling others what to do rather than doing things themselves. Our irritation with such nay-sayers leads us to issue a challenge to the DSTDers: Let us make a date 10 years from now at the turn of the century to compare the relative contributions to the study of development that have been made by our two approaches.
Correlations and causation (Wachs)
Although Wachs says he has no quarrel with the possibility that genetic factors may be associated with measures of the environment, he objects to making causal statements when correlations are used. Although we all learn that one of the first rules of statistical inference is that a correlation does not imply causation, this rule is wrong – correlations can imply causation. For example, all experiments are analyzed in terms of correlations or regressions; analysis of variance is merely a computational shortcut for regression. The issue is not the statistic, but the extent of control over variables. We cannot manipulate human genotypes as we can in selection studies of nonhuman animals in the laboratory. As in other quasi-experimental approaches, however, we can randomize one variable while we study the effects of the other. For example, parent-offspring resemblance could result from shared heredity or shared environment. How can we disentangle these two possibilities? The adoption design randomizes heredity in order to investigate the effect of shared environment by studying genetically unrelated individuals adopted together into the same family. The effects of shared heredity can be investigated by randomizing shared family environment in studies of genetically related individuals adopted apart. These correlations can be used to impute causation in the sense of decomposing phenotypic variance into genetic and environmental components of variance.
Process (Bronfenbrenner, Wachs)
Bronfenbrenner, Wachs, and several other commentators contend that finding heritability is not useful in telling us about underlying processes, “the processes through which genotypes are transformed into phenotypes,” as Bronfenbrenner puts it. Developmentalists profess interest in process more than outcome, whereas behavioral geneticists are viewed as “merely” studying outcomes on some measured traits. The word process is used in different ways. It is sometimes used to refer to mechanisms of development, the hyphen in cause-effect relationships. In this sense, behavioral genetic research addresses process to a much greater degree than most developmental research, which is usually descriptive rather than etiological. Usually, however, the process-outcome distinction refers to different levels of analysis in that process implies more basic levels of analysis. The problem with this distinction is that one researcher’s process is another researcher’s outcome. For example, an IQ test is often viewed as an exemplar of an outcome measure because it involves performance on a test. IQ tests were designed to assess cognitive processes such as reasoning and problem solving, however. In contrast, learning is viewed as an exemplar of a process, even though learning is a construct that refers to changes in measured performance over time. Although most developmentalists would view measures of learning performance as more basic than performance on psychometric measures of cognitive abilities, to a neuroscientist thinking about neurotransmitters and neuromodulators, both types of measures would appear to be molar outcomes of complex neural processes.
In summary, we do not find the distinction between process and outcome helpful. The issue is levels of analysis, and quantitative genetic methods can be applied to any level of analysis. Most exciting is the use of multivariate quantitative genetic methods to explore the etiological nexus among different levels of analysis, which as we indicated (see also Hewitt), should be the next step in research on the nature of nurture.
Model fitting (Willerman)
Willerman is perturbed by model-fitting, which represents the state of the art in quantitative genetic analyses. The first version of our target article relied on the basic data of twin and adoption correlations and scarcely mentioned model fitting. Reviewers generally felt, however, that our case would be stronger if we provided tests of significance and model-fitting estimates of parameters. In the final version we added model-fitting results when possible, but we still emphasized the basic data of twin and adoption correlations because we felt that this was the level at which the data would convince most scientists that environmental measures are influenced by genetic factors. Nonetheless, as we indicated, model-fitting analyses are helpful because they yield parameter estimates based on simultaneous analyses of data from several groups, such as identical and fraternal twins reared apart and reared together. They provide tests of statistical significance for parameter estimates, and they make it possible to test alternative models. We noted that the results of model fitting verify the conclusions reached on the basis of examining the simple correlations.
Willerman also makes a specific point about model fitting in relation to the distinction between additive and nonadditive genetic variance. As we indicated, this distinction is difficult to make empirically because essentially it is based on a pattern of twin correlations in which the DZ correlation is less than half the MZ correlation. We could just pretend that all genetic variance is additive, as is usually done when heritability is estimated from twin correlations, by doubling the difference between MZ and DZ correlations. We prefer, however, to consider the possibility of nonadditive genetic influence even though we have only a crude index of it. Schönemann & Schönemann and Duyme & Capron also criticized our use of different models in our model-fitting analyses. It is a major strength of model fitting, however, that alternative models can be compared. For example, we can compare models that consider the possibility of nonadditive as well as additive genetic variance. This is, in fact, one of the problems with Schönemann & Schönemann’s supposed “model violations”: They ignore the possibility of nonadditive genetic variance. Moreover, the point is that regardless of the specific model, evidence for significant genetic influence emerges.
Miscellaneous issues (Boomsma & Molenaar, Kendler, Wachs, Willerman)
Wachs raises the concern that heritability estimates based on data from identical twins reared apart may reflect environmental similarity because of larger ecological similarities. He says that heritability estimates are valid only if being reared in different families means being reared in totally different environments. We disagree. All that is important is that the separated identical twins are reared in uncorrelated environments, not necessarily different ones. Quantitative genetic analyses address individual differences in a population and attribute these observed differences to genetic and environmental sources of variance. Larger ecological contexts – educational opportunities shared by all twins in Sweden, for example – cannot be a source of individual differences, just as even larger environmental contexts, such as the lack of sunshine during the winter months in northern Sweden, is not a variable because it is the same for all individuals in northern Sweden.
Willerman argues that the use of data for twins reared apart may be misleading because twins reared apart have not had a cotwin in the home, and therefore cannot be affected by potential assimilation or contrast-rating artifacts. Is that not a strength rather than a weakness of the reared-apart twin design?
Hay and Kendler note that we did not emphasize the distinction between shared and nonshared environment. This is a topic of great interest to us (e.g., Plomin & Daniels 1987), but it is not a distinction that we felt was important to highlight when the point we are trying to make is that environmental measures are influenced by genetic factors.
Interpretations and implications concerning the nature of nurture
Most of the comments focused on issues of interpretation and implications of finding genetic influence on environmental measures rather than general issues concerning quantitative genetic analysis. For both interpretations and implications, some commentators felt we went too far and others felt we did not go far enough.
Interpretations about nature
Heritability and environmental measures. Other commentators, among them Hay and Wachs, are concerned about the interpretation of finding heritability for measures of the environment. What we mean by the phrase “genetic influence on environmental measures” is that genetic variance (i.e., heritability) is found for these measures. This is the way we talk about heritability for other phenotypes such as personality and cognitive abilities. Finding heritability does not indicate the process by which genetic variance occurs; the developmental process is likely to involve genotype-environment transactions of the sort Wachs describes. We certainly do not equate heritability with genetic determination. For example, what does it mean when we find that a measure of life events is heritable? There are no schlimazel (Yiddish for “crooked luck”) genes that attract life’s pies in the face. Genetic differences among children might predispose some children to be reckless sensation seekers who put themselves at greater risk for accidents, however. [See Zuckerman: “All Parents Are Environmentalists Until They Have Their Second Child” BBS 10(1) 1987.]
Hay questions our conclusion that genetic influence is significant and substantial for widely used measures of the environment. He says that “genetic estimates are small and virtually never significant at better than the 5% significance level.” Most important, as noted at the beginning of this Response, each attempt so far to replicate the findings described in our target article has shown genetic influence. The reason for the repeated appearance of the 5% significance level in our target article is simply that we chose 5% probability as a standard cut-off for significance; most of the differences are significant at much better than the 5% level. Concerning the magnitude of heritability, most of the available studies did not report heritability and the data were not analyzed using model-fitting procedures. Of those studies in which heritability estimates were reported, the average heritability estimates were 24% for parents’ ratings of their current family environment (Table 1), 26% for retrospective ratings of family environment (Table 5), 15% for social support (Table 10), and .34 for life events (Table 11). The average of these average heritability estimates is .25. Is it not significant if genetics explains 25% of the variance of these widely used measures of the environment?
Hay also notes that very large sample sizes are needed to prove differential heritability, that is, that some environmental measures are more heritable than others. Replication may be a more useful strategy, however. When we find, for example, that three studies in a row using different designs, samples, and measures suggest greater genetic influence for warmth than control, it would be silly not to pay attention to this finding. In the new replication of our results, Bouchard and McGue (1990) again find that the FES second-order control factor shows heritability of about half the magnitude of the heritability for other FES factors. Similarly, another new study mentioned earlier (Moster 1990) replicated our finding that controllable life events show greater genetic influence than uncontrollable life events.
Genetic influence on the environment per se (Kendler, Rutter, Tellegen). Rutter, on the other hand, argues that our position is not radical enough: Genetics influences the distribution of environments, not just environmental measures. Kendler also suggests evolutionary reasons for thinking about genetic influence on the environment itself. Rutter says that it is a “logical non sequitur” to say that finding genetic influence on environmental measures challenges the assumption that measures labelled as environment are in fact measuring the environment. (See also Tellegen.) What we said is that evidence for genetic influence on widely used environmental measures implies that labelling a measure as environmental does not make it an environmental measure. This is not a logical non sequitur, it is a truism or even a tautology. If genetic factors influence environmental measures, then environmental measures are not solely influenced by environmental factors. Rutter’s main point is a good one: Genetic influences could substantially affect an environmental measure, but the association between an environmental measure and an outcome could be environmentally mediated.
Jeopardy and IQ (Turkheimer & Gottesman). Turkheimer & Gottesman argue that finding genetic influence on environmental measures “weakens our already shaky confidence in the meaningfulness of traditional analyses of heritability.” On the contrary, we see Turkheimer & Gottesman’s example which is meant to cast doubt on traditional analyses of heritability, as a good example of why such analyses represent a useful first step in understanding a phenomenon.
Turkheimer & Gottesman pose the hypothetical example of children adding one IQ point for every 10 hours they watch Jeopardy. Turkheimer & Gottesman wonder who would care about heritability if such an association were found. We care. In the thousands of articles on the “effects” of television viewing, this association would be viewed as environmental: Watching jeopardy increases IQ. But what if the tendency to watch Jeopardy shows genetic influence? Turkheimer & Gottesman say that this tendency undoubtedly has a genetic component, but we would counter that this must be assessed rather than assumed, because some environmental measures show little genetic influence. Furthermore, if it is so obvious that individual differences in watching television are genetically influenced, why have the thousands of articles on children’s television viewing never mentioned genetics? Indeed, we bet that watching Jeopardy does not show genetic influence because it is too specific a response.
Nonetheless, if significant genetic influence were found for the tendency to watch Jeopardy – for example, identical twins are more similar than fraternal twins – we would also want to know something about the effect size. How much of the variance in children’s Jeopardy-watching results from genetic factors? This is heritability – merely a descriptive statistic that describes the effect size of genetic influence, just as a correlation is a descriptive statistic that describes the effect size of an association. Heritability does not tell us what processes are responsible for genetic influence, just as a correlation does not tell us what processes are responsible for the association.
If genetic influence on Jeopardy-watching is significant and its effect size substantial, then it raises the possibility of a different interpretation of the Jeopardy-IQ association. The association might be mediated genetically rather than being caused because Jeopardy-watching increases IQ. We need to assess the extent of the genetic contribution to the Jeopardy-IQ association before we can interpret the association. Turkheimer & Gottesman say that the important discovery that needs to be made is that an activity exists that can increase IQ. If the Jeopardy-IQ association is mediated genetically, however, then watching Jeopardy does not increase IQ – brighter children watch Jeopardy.
Contrary to Turkheimer & Gottesman’s interpretation, the point of the Plomin et al. (1977) reply to Roberts (1967) – that it matters whether the effects of genes are mediated through the external environment or through, say, the ribosomes – is not “that variance partitioning can be deceptive, because variance can sometimes be manipulated.” The point was to say that genotype-environment correlation cannot be attributed to genotype as Roberts wished to do, because it is a correlation that involves both genotype and environment. Genotype-environment correlation complicates the partitioning of variance, but it is not “deceptive” or “manipulated.” Neither genetic influence nor genotype-environment correlation stands in the way of programs that enable new environmental interventions. Change the mix of genetic and environmental influence, and descriptive statistics such as heritability that describe that mix will change, just as we would expect a correlation to change when changes occur in the contributors to the association.
H² = 0 (Turkheimer & Gottesman). As can be seen from the commentaries, not everyone is willing to agree with Turkheimer & Gottesman’s conclusion that “H² = 0 is no longer an interesting null hypothesis.” We are not willing to accept that conclusion either. Because genetic influence is so ubiquitous, it is more important to find phenomena that do not show genetic influence than it is to find genetic influence on yet another new measure. For this reason, we think it is especially interesting that the early results of research on the nature of nurture suggest that parental, affection shows greater genetic influence than parental control, that the quality of social support shows greater genetic influence than the quantity of support, and that controllable life events show greater genetic influence than uncontrollable life events.
Genotype-environment correlation (McGue et al.). McGue et al. argue that the terminology of genotype-environment correlation is not too narrow to encompass the nature of nurture. We agree. What we said was that quantitative genetic analyses that limit the search for genetic influences on environmental measures to traditional genotype-environment correlation are limited. We agree that at the level of process genetic influence on environmental measures needs to be phrased in terms of genotype-environment correlation, a correlation between genetic propensities and aspects of the environment. They quote from their recent Science article (Bouchard et al. 1990) concerning effective experiences, but they do not mention their good phrase, “nature via nurture” (in contrast to nature vs. nurture), that captures their view (and that of Scarr & McCartney, 1983) that genotype-environment correlation can be interpreted causally in terms of genes driving experience. Although it is tempting to lean in that interpretative direction, we think it is safer to treat genotype-environment correlation as a correlation that involves joint effects of genes and environment without assuming that variation in one causes variation in the other. In the absence of data to the contrary, it is just as reasonable to view genotype-environment correlation as incorporating bidirectional, reciprocal, developmental transactions between genetic and environmental factors.
Which environmental measures? (Duyme & Capron). Finally, we do not agree with Duyme & Capron’s statement that a measure of the environment may be submitted to genetic analysis only if it is a reflection of behaviors for which it might be possible to localize the genes. Any ostensible environmental measure can be submitted to quantitative genetic analysis. The analysis, rather than our a priori assumptions, will tell us if there is any heritable influence. Also, Duyme & Capron argue that evidence for genetic influence on SES is confounded with prenatal effects. Father-offspring resemblance in adoption studies is not confounded with prenatal effects, however; twin results do not suffer from this problem and yield similar results suggesting genetic influence.
Interpretations about nurture
The commentaries raise many issues concerning the interpretation of nurture in nature-of-nurture analyses.
Validity and reliability (Hay, Wachs). We agree with Hay and Wachs that just the fact that a measure of the environment has been widely used in the literature does not necessarily guarantee its validity or reliability. For example, we agree that videotapes of interactions between mothers and their infants may not be valid (they are reliable), but don’t we get some credit for trying to move beyond paper-and-pencil measures? Similarly, despite the HOME’s methodological problems, isn’t this observational measure a step in the right direction away from research that solely assesses parental childrearing attitudes? We can only use measures that exist, and we will gladly use more valid and reliable measures of the environment as they are developed.
Importance of environmental variance (Goodman & Stevenson, Graham, Johnson). Several commentators emphasize that the bulk of variance of environmental measures is environmental in origin. These comments suggest that we need to repeat what we said: The data suggesting genetic influence on environmental measures by no means imply that the variance of environmental measures is entirely genetic in origin. On the contrary, these data suggest that nongenetic factors are primarily responsible for variance on environmental measures. It is not news to say, however, that environmental measures are environmental in origin. The news is that widely used measures of the environment often show as much genetic influence as measures of behavior such as personality do. Graham says that we have shown ourselves to be “obviously ambivalent” about this when we say on the one hand that “nongenetic factors are primarily responsible for variance on environmental measures” and on the other hand that “genetic influence is significant and substantial on widely used measures of the environment.” Explaining something like 25% of the variance of a complex phenomenon in the social and behavioral sciences is a major achievement, even though it does not account for the majority of the variance.
Environment and measures of the environment (Graham, Tellegen). Several commentaries also addressed the distinction that we draw between the environment and environmental measures. Graham states that “surely the second should be a simple reflection of the first.” Tellegen sees our position as a “contradiction” or “sign of ambivalence” because we discuss our results operationally in terms of genetic Influence on environmental measures rather than assuming that our index of the environment is the environment itself. We wrote against the position that some critics have taken, namely, that if genetic influence is found for a particular measure, then the measure cannot really be a measure of the environment. For example, we disagree with Crusio’s argument that measures should not be called “environmental” if they reflect properties of the individual. Tellegen’s suggestion that we focus on the environment in terms of a latent variable rather than fallible measures of the environment is not to the point.
We agree with Tellegen (and Rutter and others) that the matter is partly semantic regarding how the environment is defined. But we still maintain that the traditional concept of the “environment-out-there” can show no genetic influence because it has no DNA. As we indicated in the first paragraph of our target article, the environment independent of the organism (such as the electric shock applied to the paw of a mouse) does not have DNA, whereas measures of the environment used in the behavioral sciences almost always, and perhaps necessarily, blur the distinction between environment and the organism. Surely we can agree that it is absurd to consider genetic influences on environmental factors such as the weather (temperature, pressure, cloudiness), even though individuals’ reactions to these meteorological conditions differ. Similarly, such life events as economic depression and epidemics of disease are not genetic per se. The issue is that psychological environments are not often like this. Psychological environments are not “out there,” imposed on a passive organism, but rather “in here,” experienced by an active organism who perceives, interprets, modifies, selects, and creates environments. If environment is defined “in here” as experience, then environments per se, not just measures of these environments, can of course show genetic influence.
We made what we think is a conservative decision to limit our discussion to the operational, empirical level of measures of the environment. Our thinking was this: The conceptual issue of the interface between genes and. the environment is debatable and somewhat semantic, but what is clear is that if one analyzes measures of the environment as phenotypes in quantitative genetic analyses, one finds significant and substantial genetic influence.
Other models (Tellegen, Wachs). Tellegen’s expository model depicted in his Figure 1 limits genetic influence on environmental measures to genetic Influence mediated by a particular behavioral trait such as extraversion. His model is functionally similar to the model depicted in Figure 1a in our target article, except that the usual double-headed arrow between an environmental index and behavior is replaced with two separate paths. The same can be said for the model presented in Wachs’s commentary. As we indicated, this is an appropriate model for the analysis of genotype-environment correlation for a particular behavioral trait, but it is limited to finding genetic influence on the environmental measure only to the extent that the environmental measure relates to the particular behavioral phenotype under investigation. Our environment-as-phenotype approach is more radical In that it. considers genetic influence on environmental measures regardless of their association with any particular behavioral phenotype.
Biased recall (McGue et al., Willerman). McGue et al. mention some recent work (Finkel & McGue 1990) on the reliability of retrospective reports of rearing that they interpret as suggesting biased recall. It seems likely that a retrospective report – indeed, any self-report measure – shows biases of recall. We need to understand why such processes of recall and attribution show genetic influence. The data that McGue et al. describe suggest an interesting association between neuroticism and individuals’ perceptions of family nurturance, although we cannot conclude that these data necessarily involve bias just because parents of these individuals do not show the association.
Willerman also notes several possible problems, such as low recall accuracy and reconstruction of past experiences as assessed by self-report. He suggests that adults who believe that their lives have not gone well might be more inclined to have more negative recollections of childhood. This is an empirical question about what mediates genetic influence on a measure involving retrospective ratings of the family rearing environment. Willerman might be right that genetic variance on such a measure arises for reasons of attribution and reconstruction rather than, say, personality. As indicated in our target article and discussed later in this Response, a major direction for research on the nature of nurture is the exploration of processes by which genetic variance emerges for measures of the environment. Willerman assumes that genetic influence on self-report measures of family environment is nothing but personality. This may prove to be the case, but our first effort to explore this issue empirically concludes that most of the genetic influence on family environment measures is independent of genetic influence on major dimensions of personality (Chipuer et al., submitted). Although Willerman considers only self-report measures of family environment, it is particularly impressive to us that significant heritability is found as well for objective measures of the environment such as the HOME and ratings of videotapes of mother-child interaction.
Hay also questions an aspect of the results in one of the studies involving twins reared apart; he notes that identical twins reared apart are somewhat more similar than identical twins reared together (Tables 1 and 11). Contrary to Hay’s lecture about statistical significance in the rest of his commentary, he fails to note that these differences are not significant. Furthermore, he neglects to mention that, in the same tables, data for fraternal twins do not conform to his hypothesis, and that other data from the same study do not show correlations for identical twins reared apart that exceed correlations for identical twins reared together (Tables 1 and 5).
Implications
Implications for socialization research (Rowe, Scarr, Schulsinger). Rowe and Scarr use findings of genetic influence on environmental measures and the importance of nonshared environment to cudgel socialization researchers who have ignored genetic influence for so long. More progress is likely to be made, however, by emphasizing that these findings provide an exciting opportunity to look at old issues in a new way. Schulsinger seems almost sad about these findings, suggesting that they open a Pandora’s box. We agree with his specific suggestions, such as the need for valid measures and good hypotheses. We wish Schulsinger would cheer up about these findings, however: We are confident that in the end these findings will foster more and better research on the environment.
Baumrind criticizes genetic research for not having implications for socialization researchers. For example, she says that finding genetic influence on environmental measures “offers little to the socialization researcher.” This position seems refuted by the commentaries from such socialization researchers as Bradley & Caldwell, Bronfenbrenner, Caspi, Lytton, Rutter, Tellegen, and Wachs. If, as Baumrind says, the concern of the socialization researcher is “how to help caretakers in various socio-ecological niches to nurture nature most effectively,” how does she know what that “nature” is that she proposes to nurture?
Implications for behavioral genetic analyses (Boomsma & Molenaar, Bronfenbrenner, Crusio, Hay). Crusio notes that finding genetic influence on environmental measures implies that genotype-environment correlation is important and deserves greater attention in behavioral genetic analyses of variance. Boomsma & Molenaar suggest that behavioral genetic estimates of shared environment, as meager as they are, may be inflated by genetic influence on shared environment when assessed in twin studies. This may explain why twin studies yield higher estimates of shared environmental influence than studies of adoptive siblings do. We agree with both of these points, although we suggest that behavioral genetic research that incorporates specific measures of the environment will take us farther than attempts to refine the traditional anonymous components of variance approach.
Hay recommends that multivariate genetic analyses should be conducted on environmental measures. We agree that such analyses might yield interesting results about the covariance structure of environmental measures.
Bronfenbrenner suggests that the major need for behavioral genetic analyses is not just to include any measure of the environment, but measures of reciprocal social interactional processes such as indices of mutual mother-infant responsiveness. He also makes the important recommendation that such analyses be repeated using two or more contexts such as different social groups that present contrasting conditions.
Political implications (Baumrind, Graham). For some commentators, concerns about the political implications of finding genetic influence seem to be the root cause of unease with genetics. For example, only at the end of Baumrind’s commentary do we see what seems to be motivating her strenuous attempt to discredit quantitative genetic research conceptually and methodologically rather than accepting the much simpler explanation that genetics is important:
When social problems seem intransigent, as so many do today, scientists as well as politicians turn easily to biological explanations. The thrust of the target article (whatever the motives of its authors) Is to elevate genetic determinism as an explanation for human behavior. Cultural determinism and genetic determinism both undermine the attribution of personal responsibility to the individual as a moral agent.
Graham also seems upset by the political implications of these findings. We agree with Graham that finding genetic influence on environmental measures is important without making any claims for its practical value. Now that this issue is on the table, however, we will expose our old-fashioned philosophy of science. We believe that finding genetic influence is compatible with a wide range of social action, including no action at all. Values help us decide what we want to do with such knowledge, and we believe that on the whole better decisions can be made with knowledge than without. For example, finding genetic influence on IQ by no means implies that “them what gots, gets.” Depending on our values, we could argue that scarce educational resources should go to those who most need them to function adequately in our society.
As another example, Graham asks whether finding genetic influence on children’s television viewing should discourage us from interventions to cut down the time children spend watching television. It should not, but for reasons other than the reason given by Graham. He suggests that because genetic factors account for only a quarter of the variance in television viewing, its effect is too weak to carry practical implications. We suggest that decisions to intervene to discourage children’s television viewing depend on our values, not on the magnitude of genetic influence. Graham’s value is that television viewing is bad for children. Our value on this topic happens to be much more extreme: We hate television with a passion, and would blind all the one-eyed monsters in the world if given the chance. Our proposed violent intervention comes from our own values, however, and is unrelated to whether or not genetic factors affect individual differences in television viewing. Other people have different values. For example, an “individuals’ rights” perspective might be that television viewing is a victimless crime that one ought to be able to perpetrate on oneself if that is what one wants to do. Regardless of what our values lead us to recommend, knowledge of etiology might be helpful. For example, given Graham’s implied value that television viewing is bad for children, it would be helpful in his hypothetical television intervention studies to consider the possibility that some children are at greater “risk” for television viewing than others and to target interventions specifically for these children, even if only a quarter of the variance in television viewing is accounted for by genetic factors.
Directions for future research on the nature of nurture
Simonton’s case study of genius is an example of the heuristic value of research on the nature of nurture. In 1988, Simonton wrote a book on genius that proposed an interesting “chance-configuration” theory. In a chapter discussing the origins of genius, he considered parental loss and orphanhood, birth order, cultural enrichment, role models, formal education, and the Zeitgeist. Heredity is mentioned only in passing on a single page. On the basis of Simonton’s present commentary, however, we can hope that another book of genius is budding that considers possible genetic origins of genius and of associations between socialization factors and genius. In this section, we highlight suggestions that commentators have made for future research on the nature of nurture. The major categories of suggestions involve conceptualizations of the environment, measures of the environment, and multivariate analyses. We were especially pleased with the commentary by Waldman & Weinberg because it was entirely forward-looking. We agree wholeheartedly with their exhortation (and Wachs’s) for greater interaction between behavioral geneticists and environmentally oriented researchers. We agree with all four directions they outline: development of reactive and active measures of environmental interactions; identification of method, source, and target factors; investigation of mediators and moderators; and consideration of developmental changes. Their suggestions for research on moderators and consideration of development changes take on special significance because these ideas were not discussed in our article nor in the other commentaries.
Conceptualizations of the environment
Genotype → environment processes (Scarr). Scarr proposes two principles that point to future directions for research and elaborate her theory for genotype → environment effects, a theory that emphasizes that people make their own environments (Scarr & McCartney 1983). Her first principle is that the environment is best construed as an array of opportunities for behaviors to develop. For example, the family can be seen as a diverse opportunity structure for its members.
Several implications of this principle were considered in our target article and the commentaries. For example, we are interested in the possibility that genetic influence on the ways in which organisms interact with their environments might be responsible for the ubiquitous genetic influence found for behavior. Kendler indicates a similar interest: Genes may alter risk for psychiatric disorders by influencing pathogenic or protective features of the environment. Socha extends this idea even further by considering environmental influences on DNA itself, that is, transcriptional and translational changes in responses to the environment. For example, a very active area of molecular genetic research involves DNA responses to environmental stress (e.g., Scandalios 1990).
Scarr’s second principle is that environments have nonlinear effects on behavioral development. Specifically, she suggests that low-quality environments affect development, but environments in the adequate to superior range have little effect beyond genotype-environment correlation. Bradley & Caldwell suggest that both genetic and environmental influences are most powerful at the negative extreme. Future research would do well to investigate these testable hypotheses about etiological differences between the low extreme and the rest of the distribution. A related hypothesis is that results could differ, not just at the extremes of the distribution for a particular population, but in such atypical populations as the severely handicapped (Bradley & Caldwell), in different populations (Duyme & Capron), or in different cultures such as peasant families living in the Peruvian Andes (Baumrind). Mowe reminds us that cross-cultural differences do not lead to simple interpretations.
Parental effects and child effects (Lytton). Lytton presents a very interesting hypothesis concerning the finding that parental warmth appears to show more genetic influence than parental control. His observational work suggests that, for warmth, the direction of effects is from child to parent, whereas for control the direction is more from parent to child. He also suggests that parental control might be shown to be influenced genetically in a twin study of parents, which is in fact suggested by our SATSA findings (Plomin et al. 1989). We agree with Lytton’s suggestion that more research is needed to distinguish between parental effects and child effects. His is one of the only studies of this sort, which is why we referred to it as a pioneering study that is an exemplar of the type of research that is needed.
Moos’s taxonomy (Caspi). Caspi describes Moos’s (1973) sixfold taxonomy, which classifies aspects of the environment. Although, as Caspi indicates, there is as yet little agreement about which aspects and levels of the environment should be analyzed, such classifications will be useful in designing research on the nature of nurture. We agree with Caspi’s provocative argument that we need to keep measures of person and environment as distinct as possible from each other if we ever hope to integrate them explicitly in an interactional theory. Interactionists will not like this position, however.
Objectivity and subjectivity (Tellegen). Tellegen’s distinction between objective-consensual and subjective-experiential environmental latent variables is a good one. It can be viewed as a subset of the suggestion by Waldman & Weinberg to consider method, source, and target variance. These issues could be investigated in a multi-method mode by modeling a common latent trait involving different rating sources including self-report (i.e., a “consensual” common factor). Variance unique to each rating source could then be considered; variance unique to the self-report measure is one way to operationalize the subjective-experiential notion. This objective-subjective distinction is likely to prove useful because genetically influenced processes that can contribute to these two types of environmental measures differ. The subjectivity/objectivity dimension does not so far appear to be related to the extent of genetic influence on environmental measures, however. (See our Figure 2.)
Models of genotype-environment correlation (Tellegen, Wachs). The genotype-environment models proposed by Tellegen and Wachs are helpful in extending our thinking about the interface between nature and nurture. As mentioned earlier, we see these models as similar to the traditional genotype-environment correlation models presented in their simplest form in our Figure 1a. In our view, such models are limited to finding genetic influence on an environmental measure only to the extent that the environmental measure relates to the particular behavioral phenotype included in the analysis. Nonetheless, models of this sort represent one way to think about the organismic phenotypes that contribute genetic variance to measures of the environment. We return to this issue in the final section on multivariate analysis.
Environmental measures
Experience (Bronfenbrenner, Caspi, McGue et al., Rutter). Several commentators, including McGue et al. and Rutter, agree with our point that we need better measures of experience, the subjective environment. Caspi, however, finds the distinction between subjective and objective approaches to the environment unsatisfying and discusses an interesting alternative approach that attempts to assess the stimulus context of situations (Block & Block 1981).
Reactive and active measures (Bradley & Caldwell, Caspi). We also emphasized that we need measures that move beyond the passive model of the individual as merely a receptacle for environmental influence, to measures that can capture the individual’s active selection, modification, and creation of environments, a point that Caspi extends in his commentary and in his longitudinal research on social selection. (See also McGue et al.) Bradley & Caldwell remind us that we also need measures of reactive genotype-environment correlation that assess the reaction of others to genetically-influenced characteristics of the individual. Caspi suggests that this is a better way to think about subjective aspects of the environment.
Behavioral/nonbehavioral and person/physical measures (Bronfenbrenner, Graham). Graham suggests that researchers should distinguish between behavioral and nonbehavioral measures of the environment, such as the number of persons per room, because he believes that the genetic contribution to the latter is not measurable. The distinction between behavioral and nonbehavioral measures might be useful, although most environmental measures used by behavioral scientists are not easily divided between those that are behavioral and those that are not. There is probably a great deal of overlap between Graham’s distinction and Bronfenbrenner’s suggestion that we consider personal and physical processes.
Multivariate analyses
Many commentators underscored a point mentioned at the end of our article: A major direction for research at the interface between nature and nurture is multivariate investigation of the antecedents and sequelae of genetic influence on measures of the environment. We stated that multivariate genetic analyses of the phenotypic covariation between behavioral and environmental measures are needed to determine the extent to which such behavioral measures can account for genetic influence on environmental measures. Concerning sequelae, it is possible that associations between environmental measures and behavioral outcomes – for example, life events and depression – are also mediated genetically, if both are influenced genetically. As Rutter notes, a measure of the environment can be influenced genetically, but its association with an outcome could be mediated entirely environmentally.
Such multivariate analysis is the title and main theme of the commentary by Hewitt. Johnson suggests that the liberalism-conservatism dimension that pervades personality and shows substantial genetic influence could be an important mediator of genetic influence on experience. Johnson also proposes a novel strategy for finding mediators of genetic influence on environmental measures. He suggests starting with a strongly genetically influenced trait of parents and investigating how experiences differ for individuals high and low on the trait.
Several commentators criticize our paper for not discussing the processes by which genetic factors affect measures of the environment (Rutter, Socha, Tellegen, Wachs). As mentioned earlier, we felt that the target article would have greater impact if it focused on the phenomenon of genetic influence on environmental measures, which could be solidly documented, rather than speculating about the sources or sequelae of this genetic influence about which very little is as yet known.
Since writing our target article, we have worked on a paper that uses a trivariate quantitative genetic model to investigate the extent to which genetic influence on environmental measures can be accounted for by genetic influence on extraversion and neuroticism (Chipuer et al., submitted). We find that most of the genetic variance on environmental measures is independent of genetic variance on these two major dimensions of personality, suggesting that these personality dimensions do not by themselves explain the puzzle of genetic influence on environmental measures.
Molecular genetics
Several commentators (Baumrind, Kendler, Lytton, and Socha) predict that molecular genetic studies will help settle questions about genetic influence on behavior. Imagine being able to identify behavior- or environment-relevant DNA variation directly in individuals rather than resorting to indirect estimates of a genetic component of variance derived from twin and adoption studies. Advances in molecular biology are well on the way to making this fantasy a reality (Plomin 1990). It was only 10 years ago that the now standard techniques of the “new genetics” of recombinant DNA were first employed to identify genes responsible for disorders. We predict that 10 years from now, at the turn of the century, molecular genetic techniques will have revolutionized human behavioral genetics. Kendler’s caution is well taken, however: As genetics moves toward reductionist molecular models, it is also important to move out into the environment to understand the inevitably complex interplay between our genes and the environment around us.
In closing, we thank the commentators who contributed their ideas about interpretations and implications and, especially, about directions for future research. We hope that this discussion will stimulate research that leads to new measures of the environment as well as mechanisms to explain genetic influence on environmental measures.