In this book, Richard D. Fuerle (2008) first rigorously expounded upon the evolutionary principles and then provided evidence that Africans lack many essential modern traits, and finally that the Out of Africa (OoA) theory is flawed. Fuerle speculated that if Africans were extinct long ago and archeologists would find their remains, Africans could have been classified as a different species. The book contains a mix of very good and somewhat bad materials. Importantly, the cited evidence supporting the proposition that modern humans originate from Eurasia (and not Africa) were often weak, sometimes even misleading, despite raising numerous and interesting issues regarding OoA’s assumptions. It is yet obvious that OoA is much less convincing than often assumed. There are many reasons for its weakness, e.g., OoA’s haplotype predictions not fitting the observed genetic patterns (Huang, 2025a) and OoA’s reliance on implausible assumptions such as the molecular clock (including the relaxed clock) and infinite site model (Huang, 2025b), and the independent evolution of modern traits in Asia (Liu & Wu, 2022). The violation of the molecular clock assumption is particularly egregious because it implies that fossil calibration dates may be incorrect, admixture event dates are distorted, phylogenetic trees mislead about evolutionary rates.
This review will complete and correct the arguments put forth by Fuerle.
CONTENT
SECTION 1 – Introduction
Chapter 1 – A Story of the Origin of Humans
Chapter 2 – Early Humans
Chapter 3 – DNA
Chapter 4 – Evolution
Chapter 5 – Selectors
Chapter 6 – Neoteny
Chapter 7 – Genetic Distance
Chapter 8 – Evolutionary Psychology
SECTION 2 – Traits of Living Populations
Chapter 9 – Hard Tissue
Chapter 10 – Soft Tissue
Chapter 11 – Reproductive Strategy
Chapter 12 – Behavior
Chapter 13 – Genes
Chapter 14 – Intelligence
Chapter 15 – Civilizations and Achievements
Chapter 16 – Primitive Traits
SECTION 3 – The Out-of-Africa Theory
Chapter 17 – Fossil Skulls
Chapter 18 – Modern Behavior
Chapter 19 – MtDNA
Chapter 20 – Population Differences in MtDNA
Chapter 21 – Nuclear DNA
Chapter 22 – Replacement
SECTION 4 – The Out-of-Eurasia Theory
Chapter 23 – The Bipedal Apes
Chapter 24 – The Origin of the Eurasians
Chapter 25 – The Neanderthals
Chapter 26 – The Origin of Africans
Chapter 27 – The Origin of Asian Aborigines
SECTION 5 – Policy
Chapter 28 – Homo Africanus
Chapter 29 – Miscegenation
Chapter 30 – Hybrid Vigor
Chapter 31 – Segregation
Chapter 32 – Eugenics
Chapter 33 – Re-Classifying the Left
Chapter 34 – Egalitarianism
Chapter 35 – Individualism
Chapter 36 – Morality
Chapter 37 – Which Way Western Man?Section 1 – Introduction
Egalitarianism mostly infected the West, destroying careers, bankrupting companies, wasting trillions of dollars. Scientists “disappear” if they do not reach the “right” conclusions. Given the assumption that all the races are genetically equal, they could not have diverged long ago and therefore the origin of modern man must be recent and all living human beings are fully modern. An interesting observation is that if everyone is genetically equal, then the cultures they create should also be equal. This implies that cultures should be equally respected and people of all cultures should not only be able to live peacefully together in the same territory.
Chapter 1 – A Story of the Origin of Humans
The origin of humans began around 60 million years ago in Southeast Asia, where early primates (prosimians) lived in trees. Some developed an upright posture, using their hind legs for support and front legs for grasping. As some grew larger, they adapted to swinging with their arms (brachiation), leading to longer arms and reduced tails. Although the evolutionary pressures leading to tail loss are still unknown (Tojima, 2021), this tail-loss evolution is the result of the insertion of an Alu element in the genome of the hominoid ancestor (Xia et al., 2024). Around 25 million years ago, tailless brachiators became apes, spending more time on the ground. These apes developed different walking styles: palm-walking in Eurasia and knuckle-walking in Africa (Begun, 2016, p. 218), although it is unclear whether knuckle-walking evolved independently in the 2 African ape lineages, gorillas and chimpanzees (Begun, 2016, pp. 218-220; Kivell & Schmitt, 2009; Williams, 2010). A complex terrestrial environment positively selects for intelligence and brain size.
Some Eurasian apes adapted to marshes and rivers, occasionally walking on two feet (bipedalism), which allowed them to travel farther, use tools, and carry food, while also reducing heat exposure (see also, Harcourt-Smith, 2015, pp. 1945-1959). This shift, around 10 million years ago, marked a big step toward humanity, but only because of the continued pressure to gather food (Corballis, 1993, pp. 53-54). Bipedalism improved predator detection, and carrying infants influenced brain specialization, leading to right-handedness. This simple act of carrying the baby with one arm may have profoundly affected the human brain. Because the left ventricle of the heart makes the loudest sound and babies are calmer when they hear the heartbeat they heard in the womb, most women (even today) carry their babies on the left side (Calvin, 1991). Women, like men, used their “free” right arm to throw stones at prey and predators, and those women whose left brain side (which controls the right arm) was more skillful in performing precise throws had an advantage. That bipedalism led to cerebral and strength asymmetry, shaping thusly walking and throwing, seems to be a key evolutionary factor that formed the human body (Corballis, 1993, pp. 99-104, 193-195, 207; Longman et al., 2020). Thus, humans became predominantly right-handed, and their brains became more asymmetrical, making the brain more specialized and sophisticated. Humans are the only primates that are predominantly right-handed. It is more likely however that right-handedness may have arisen because of an association between manual gestures and vocalization in the evolution of language (Corballis, 2003, 2009).
The evolution of hominin brain size had to select against greatly expanded pelvis (and the large body sizes it would entail) because further fetal brain expansion is constrained by the limits of maternal metabolism (Dunsworth et al., 2012; Lieberman, 2011, pp. 222-223). Natural selection favored a shorter gestation period and less developed neonates to accommodate both locomotion and encephalization. It implies that narrower birth canals in women facilitated more efficient walking but required infants to be born less developed (Haeusler et al., 2021), necessitating extended care. Walking uses less energy if the legs are closer together, and women with a narrow birth canal (and therefore closer legs) survive better, and although brain growth is delayed, it has its strongest growth after birth (Corballis, 1993, pp. 69-70; Gómez-Robles et al., 2024).
Despite multiple studies showing that wide pelvises are just as energy efficient as narrow pelvises in bipedal locomotion (Mitteroecker & Fischer, 2024), there are some evidence that this functional tradeoff in the human pelvis is real even if the once-proposed “solution” of early birth in terms of gestation length is not (Grunstra et al., 2023; Haeusler et al., 2021). There is some evidence that birth canal size varies across races (Rushton & Rushton, 2003) and is relatively wider across the hips in colder climates (Betti & Manica, 2018) as well as in high-latitude populations (Kurki, 2013; Betti, 2017).
Bipedal apes migrated to Africa, outcompeting other great apes except chimpanzees and gorillas who retreated to more isolated areas, and by ~4 million years ago the bipedal ape has become Australopithecus, the last bipedal ape. Then, competition in the tropics pushed some groups northward into colder, seasonal climates, which required northern adaptation, which in turn selected for greater intelligence due to limited food availability. Around 2.5 million years ago, the combination of efficient bipedal walking, free use of hands, and greater intelligence had paid off big time and the ape had become man. Around 2 million years ago, some australopithecines had significantly larger brains, marking the birth of the Homo genus. There are some inaccuracies in Fuerle’s dates because the global climate shift toward a cooler world, marking the origin of the genus Homo, occurred 2.5 millions years ago (Dunsworth, 2010; Timmermann et al., 2022).
Survival in harsher northern climates selected for traits such as intelligence and brain size and it also favored future planning over impulsivity. The relationship between the sexes also changed (Frost, 2019). Women could no longer gather food and had to rely on men, and those men who committed to a single woman passed on their pair-bonding genes. It is also possible that cooperative foraging for sources of meat favored more sophisticated forms of communication and by the same token the development of specialized skills within the social group. These factors might explain the increase in brain size (Coon, 1962, pp. 78, 86; Corballis, 1993, pp. 65-66; Grabowski et al., 2023).
Homo erectus spread across Africa, Europe, and Asia, replacing Australopithecus. Erectus expanded territory until further migration required overcoming other erectus populations. Over time, erectus continued to evolve, each population becoming better adapted to its unique environment, and they became genetically distinct. In West Asia and Europe, a separate lineage became Neanderthals about 350,000 years ago. In East Asia, erectus adapted to the cold climate, developed fire, and evolved into Homo sapiens 200,000 years ago. Eventually, Homo sapiens refined intelligence and skills, completing the transition to Homo sapiens sapiens 150,000 years ago.
Chapter 2 – Early Humans
The classification of human fossils is challenging because species evolve gradually, and cranial capacity varies between individuals and sexes, making it difficult to trace evolution linearly. And fossil locations do not even prove origins.
The first known member of the genus Homo is Homo habilis, which lived between 2.5 and 1.8 million years ago. Habilis had a small cranial capacity (500-800 cc), a primitive face, and a less protruding jaw than its ape-like predecessors. It likely had rudimentary speech capabilities and was the first hominid to incorporate meat into its diet. Habilis may have descended from a gracile bipedal ape.
Homo ergaster, an early African Homo erectus, lived 1.9-0.6 million years ago and had a cranial capacity of 700-880 cc. Ergaster may have used fire and made tools, though its tools showed little improvement over a million years. A nearly complete skeleton, “Turkana Boy,” found in Kenya, indicates that ergaster could swing its arms while walking or running and would have grown to over 6 feet tall. It seems perhaps dubious that Ergaster originated in Africa (Dennell & Roebroeks, 2005).
Homo erectus lived across Africa, Europe, and Asia, with a cranial capacity of 750-1225 cc. It had a prominent jaw, thick brow ridges, and no chin, but was taller and had a larger brain than its predecessors. Early erectus had an average cranial capacity of 900 cc, while later erectus averaged 1100 cc. Homo georgicus, found in Georgia, had a cranial capacity of 600-800 cc and shared similarities with habilis and ergaster, though it was more gracile and adapted to colder climates.
Homo antecessor, dated to 780,000-857,000 years ago, had a cranial capacity of 1000-1150 cc and showed signs of cannibalism (Saladié & Rodríguez-Hidalgo, 2017). It was robust, with an occipital bun and no chin, and may have been a precursor to Homo heidelbergensis (although this is disputed; see, Stringer, 2012) and Homo neanderthalensis. Heidelbergensis, living between 800,000 and 200,000 years ago, had a rounder skull than erectus, with a cranial capacity of 1200 cc, and may have been an ancestor of Homo sapiens in Africa and of Neandertals in Europe (Mounier et al., 2011; Timmermann et al., 2022).
Neanderthals, with a cranial capacity of 1450 cc, lived from 350,000 to 24,500 years ago in Europe and the Middle East, although many figures indicate they went extinct 40,000 years ago (e.g., Lieberman, 2011, p. 543; Wells, 2016, p. 177). Their barrel-shaped chest (Bergmann’s rule), and short, stocky hands, fingers, and feet (Allen’s rule) were adaptations to the cold, and due to the lack of sunlight in the north, they would have had white skin and may also have been hairy. Neanderthals had advanced tools and were skilled hunters, though their diet did not follow the typical primate pattern of robust herbivores and gracile carnivores. Importantly, they had modern speech and language (Dediu & Levinson, 2013, 2018).
Archaic men (Hs) first appeared around 200,000 years ago. Modern men (Hss) emerged around 160,000 years ago, with an average brain size of 1,350 cc. They had smaller teeth, gracile skeletons, and prominent chins. Cro-Magnons were the immediate predecessors of modern Caucasians and lived in Europe between 40,000 and 10,000 years ago. They were slightly more robust than modern humans and had larger brains, though their skulls were thicker. Cro-Magnon culture introduced sophisticated tools, art, and cave paintings, marking a significant cultural advancement. The Cro-Magnons’ flattened eye sockets, an adaptation to cold climates, also appeared in some North African skulls possibly due to interbreeding during migrations.
Chapter 3 – DNA
Humans have 23 pairs of chromosomes, totaling 46, with one set inherited from each parent. Chromosomes consist of DNA strands wrapped around proteins called histones, which help unwind DNA for reading. DNA consists of nucleotide sequences (A, C, G, T), similar to a computer code, which determine gene function. The genes are DNA segments that code for polypeptides, which form proteins responsible for traits (phenotypes). Less than 2% of the human genome codes for proteins. While all humans share the same genes, different versions (alleles) of these genes result in variations like eye color. Alleles can be beneficial, neutral, or harmful, and new alleles arise through mutation or interbreeding. Beneficial alleles spread if they enhance reproductive success, while harmful ones are eliminated, though they may reappear. It should be noted however that alleles may have context-dependent fitness, e.g., sickle-cells are harmful among homozygotes but protective among heterozygotes (Luzzatto, 2012).
Expanding populations acquire alleles (because there are more people in whom mutations can occur), and declining populations lose them (because people with unique alleles, even if they are not harmful, die without leaving descendants). An example is the loss of alleles (and thus, genetic diversity) among Eurasians after large numbers died during the Ice Ages. Generally, an allele that increases reproductive success is unlikely to be lost. If an allele is widely expressed in a population, then it must increase the reproductive success of that population in its current environment. However, an allele that has been only weakly expressed for a certain period does not increase reproductive success, or increases only when it is weakly expressed and becomes harmful when it becomes too common. The number of alleles in a population doesn’t necessarily indicate its age, since many alleles may have been acquired through interbreeding with other populations rather than through mutations, as is the case with archaic alleles conferring an evolutionary advantage (known as adaptive introgression) on the recipient population due to faster rate of adaptation (Racimo et al., 2015, 2017). Thus, ancient alleles in one population may not imply ancestry over another because environmental stability or interbreeding can preserve these alleles.
DNA exists in two forms: nuclear DNA (in chromosomes) and mitochondrial DNA (mtDNA), which provides cellular energy. Nuclear DNA is a double helix with two strands, one from each parent, and contains both coding (exons) and non-coding (introns) regions. mtDNA, inherited almost exclusively from the mother (Ferreira & Rodriguez, 2024), is a single-stranded ring with no repair mechanisms, leading to a higher mutation rate. Nuclear DNA encodes most racial traits, while mtDNA rarely influences them. During fertilization, only the sperm’s nuclear DNA enters the egg, as mtDNA in the sperm is typically destroyed. While mtDNA is maternally inherited, the Y chromosome is paternally inherited.
Chapter 4 – Evolution
Humans share 98.7% of their DNA with chimpanzees, but differences between sexes are greater due to the smaller Y chromosome. This does not mean that men and women are closer to chimpanzees than to each other (a conclusion one would have to accept if we apply Lewontin’s fallacy). In fact, differences in how DNA sequences are read and assembled have a greater effect than differences in the DNA itself. If one compares total genomic divergence however, Y chromosome is much more divergent between humans and chimpanzees (Hughes et al., 2010). And if one considers gene level instead of base pair level, men and women are much more similar.
Darwin’s theory of evolution can be summarized as a syllogism: if individuals in a population have heritable traits that differ and affect reproductive success, traits enhancing reproduction will become more common. Evolution requires variation, and mutations provide the raw material, but only beneficial mutations spread in a changing environment.
Generalized vs. specialized: Species can be generalized (adaptable to varied environments) or specialized (adapted to a specific niche). Raccoons, rats, and cockroaches are generalized species; the koala eats only eucalyptus leaves, and many parasites live only at the expense of a single host species, making them specialized. Generalized species, like humans, are more resilient to environmental changes. Humans are highly generalized, capable of living in diverse environments and using technology to overcome physical limitations (Nair, 2014). For instance, they can thread a needle, swing a club, or play a piano concerto. There is obviously a trade-off between generalization and specialization (Kassen, 2002). A ‘generalist’ can do more things, but each one less efficiently. Specialization allows efficient exploitation of a niche but increases vulnerability if the niche disappears. Importantly, environmental heterogeneity (especially spatial variation and to a lesser extent temporal variation) can maintain higher quantities of genetic variation (Kassen, 2002).
The evolutionary rules can be summarized as follows:
1. Cumulative evolution. Evolution builds on existing traits. New traits emerge by modifying what already exists, and organisms must remain reproductively successful at every stage. Complexity increases over time. Exaptation (trait that was originally selected for one function but is later utilized for a different function) is indeed common (Frenkel-Pinter et al., 2022).
2. Addition over subtraction. The evolution of new traits is more likely to occur by adding alleles, copies, and regulations to an existing genome rather than by removing them (Magadum et al., 2013). A new trait can emerge when a new allele is expressed, duplicated, or when a gene regulator modifies allele expression. These changes provide raw material for evolutionary innovation, including exaptation, where existing traits are co-opted for new functions (Gould & Vrba, 1982). Whole-genome duplications (WGDs), as seen in Paramecium, generate thousands of gene copies, most of which are lost unless functionally advantageous, resulting in phenotypic innovation and increased morphological complexity (McGrath et al., 2014). Traits are lost only if they become disadvantageous, as seen in cave fish losing their eyes; the fish that become trapped in a cave can no longer exploit a sunlit niche, so eyes become an unnecessary cost, and fish that invest fewer resources in their eyes gain an advantage. Evolution by gene loss has been traditionally underestimated however (Albalat & Cañestro, 2016; Olson, 1999).
3. Generalized > Specialized > Extinction. Populations tend to evolve from generalized to specialized. Specialization can lead to extinction if the environment changes (see, e.g., Botero et al., 2015), though some species may become more generalized through neoteny (making them better able to migrate out of warm climates) or interbreeding. Researchers noticed recent declines in specialist species, related to disturbances to habitat and climate (Clavel et al., 2011), although some observed that ecologically intermediate species among these two extremes are the most seriously affected ones by recent environmental changes (Habel & Schmitt, 2012). Besides, species threatened by local extinction may nonetheless persist in unstable, variable environments using various strategies (Holt, 2009, p. 19662).
4. Environment drives specialization. Stable environments favor a population that specializes to exploit a niche in this environment, while changing environments favor generalized ones. Tropical and polar regions, being stable, host more specialized species. This is because fluctuating environments tend to select for plastic genotypes (a characteristic of generalist populations), which suffer fewer and lesser demographic bottlenecks despite steep fluctuations in the environment (Gomez-Mestre & Jovani, 2013). The interesting outcome emerging from such a model is that: 1) plastic populations have higher genetic variation because plasticity shields a broader range of genotypes from purifying selection by allowing them to express well-matched phenotypes, 2) plasticity reduces the effect of genetic drift as a consequence of maintaining greater population sizes (i.e., by reducing population bottlenecks). An important caveat is that very fast or unpredictable environmental change can make it impossible to predict the environment, causing plasticity to be selected against (Sekajova et al., 2023), and making bet-hedging the best strategy (Tufto, 2015; Wells, 2016, p. 169).
5. Genetic variation. Specialized populations have less genetic variation than generalized populations (Li et al., 2014; Pasinelli, 2022) although there are cases which go against this principle, making genetic diversity prediction quite challenging (Trense et al., 2021). Individuals who deviate from the most efficient traits in a specialized population are more likely to be negatively selected than those who deviate in a generalized population because the specialized population lives in a more stable environment. Highly specialised species don’t rely on gene flow between populations to maintain fitness, and their survival depends more on habitat quality (Habel & Schmitt, 2012). On a related note, lower maximum genetic diversity results in higher fitness traits because there are more common alleles or good alleles becoming homozygous (Huang, 2025b).
6. Evolutionary pace. Specialized populations evolve more slowly due to limited genetic variation (there are fewer traits and alleles available for selection) when the environment changes. Generalized populations are more likely to emerge in a changing climate due to their higher phenotypic plasticity (Gomez-Mestre & Jovani, 2013; Sekajova et al., 2023), and humans are more likely to have evolved (at least in their later stages) in a temperate region rather than in the tropics. Moreover, plasticity can evolve rapidly after colonization of a new environment in response to changing niche use (Svanbäck & Schluter, 2012).
7. Carrying capacity. Specialization increases a population’s carrying capacity by efficiently exploiting a niche. Thus, by specializing, a population can increase in number and, consequently, the rate at which mutations enter the population, which may allow it to evolve more quickly. Humans, however, increase carrying capacity through technology rather than physical specialization (Ellis et al., 2020).
8. Energy and biomass. More usable energy in an area leads to greater biomass and more species, either directly or indirectly (Ali et al., 2019). Tropical regions, with abundant sunlight, have the highest biomass and species diversity (Dyola et al., 2022). A hierarchical framework reveals that long-term energy availability, combined with stable warm temperatures and large historical habitats, shapes ‘evolutionary arenas’ where sustained speciation occurs (Jetz & Fine, 2012).
9. Reproductive strategies. In high-biomass environments (like the tropics), populations adopt r-oriented strategies (more offspring, less care), while K-oriented strategies (fewer offspring, more care) prevail in less abundant environments. It is worth noting that long-lived, low-fecundity K-strategist species tend to be genetically less diverse than short-lived, highly fecund r-strategist species (Romiguier et al., 2014).
10. Trait optimization. Traits evolve until they reach an optimal state for the environment. When each trait of every individual reaches its optimum, the population is in equilibrium with its environment (though such a condition is rare). The further a species is from its optimal state, the faster it will evolve or the faster it will go extinct. Thus, after an environmental change, evolution will be rapid at first and then gradually slow as equilibrium is approached (Orr, 1998). Indeed, traits tend to remain near their optima due to stabilizing selection (Estes & Arnold, 2007; Pritchard et al., 2010).
11. Trait origins. Traits are most likely to originate where they are most prevalent, as they provide a reproductive advantage in that environment.
12. Behavior drives evolution. Behavioral changes precede genetic changes. Individuals can alter their behavior to acquire resources more efficiently and, consequently, better mates. If those who exhibit these behaviors have greater reproductive success, a subset of this group possessing the anatomy and physiology that further facilitate the new behavior will be selected (see, e.g., Heyes, 2012, p. 2093). While evolving in a new environment, organisms can change their phenotype (i.e., adaptive phenotypic plasticity) and/or modify their environment (e.g., niche construction), and thereby affect selection acting on themselves and other species (Laland et al., 2015, 2016).
13. Time and catastrophes. Over time, populations accumulate genetic variation through mutations. Catastrophes reduce variation by eliminating alleles.
14. Isolation and inbreeding. Isolated populations become more homozygous (inbred), increasing the expression of recessive alleles. Because the longer a population has been isolated, the more it will be free of disadvantageous recessive alleles and the greater will be the percentage of its expressed alleles that are recessive (especially advantageous alleles), and conversely. The combination of Rule 13, i.e., mutations introducing new alleles and therefore increasing diversity, and Rule 14, i.e., selection removing less beneficial alleles, may increase genetic variation but primarily in advantageous alleles. On one hand, natural purging (i.e., the natural selection during which inbreeding exposes and removes these recessive deleterious alleles) can reduce the negative effects of inbreeding depression under condition of slow inbreeding and competitive environments and kin avoidance, dispersal, and resource scarcity had been driving human evolution, on the other hand, deliberate purging (e.g., through subdivided breeding lines or selective inbred matings) carries some risks (e.g., drift load, diversity loss) and exhibits variable success (Hedrick & Garcia-Dorado, 2016). Although purging is particularly effective against strongly deleterious alleles, it is less effective for weakly deleterious alleles in very small populations, because selection is ineffectual (Robinson et al., 2023; see also, Glémin, 2003).
There are six primary mechanisms through which the genomes of individuals in a population can be modified, leading to changes in their descendants: mutation, epigenetics, isolation, hybridization, recombination, and selection.
1. Mutation. Genetic changes occur when DNA in germ cells (eggs or sperm) is altered by factors like cosmic rays, high temperatures, or mutagens. Non-coding DNA (“junk DNA”) can also mutate and become coding DNA, potentially affecting traits in the next generation. DNA can also be modified by viral or bacterial genetic material, or through duplications and rearrangements of DNA sections. Over time, the least vital DNA accumulates the most mutations. Pseudogenes (i.e., which lost their ability to code) evolve very rapidly and are mostly subject to no functional constraint (Graur et al., 2013).
2. Epigenetics. Gene regulators, which control access to DNA, can be modified by environmental changes and passed to the next generation (see, e.g., Heard & Martienssen, 2014; Skinner, 2015) and even later generations by affecting a pregnant female, its fetus and the germ cells inside the fetus (Skinner, 2014). These regulators determine when, how often, and which parts of DNA are read. Histones, for example, unwind DNA for reading, while chemical groups like methyl can block DNA from being read. Though most methylation marks are erased during embryonic reprogramming, critical regions (e.g., imprinted genes) resist erasure, allowing stable epigenetic inheritance (Messerschmidt et al., 2014). Regulators evolve faster than DNA itself, making them a key driver of evolution (Borneman et al., 2007; Wray, 2007). Differences in gene regulators, rather than DNA, likely account for significant variations between species and races.
3. Isolation. When populations become isolated, inbreeding increases, making it easier for rare, advantageous alleles (especially recessive ones) to spread, and although inbreeding typically reduces fitness by threatening survival (Keller & Waller, 2002), inbreeding has no effect on fitness in some other organisms (Ekblom, 2000). Isolation can also help eliminate harmful alleles from the gene pool more quickly, such as inbreeding depression (Charlesworth & Willis, 2009; Keller & Waller, 2002), as inbreeding increases the likelihood of inheriting two copies of the same allele. While small, isolated populations are more susceptible to loss of genetic diversity and have increased susceptibility to environmental stressors (Kirk, 2024), populations with lower genetic diversity may depend more on cultural and cognitive evolution than on biological evolution to adapt to changing environments (Huang, 2025a). Besides, highly specialised species can be sustainably conserved through the maintenance of suitable habitats regardless of their degree of isolation (Habel & Schmitt, 2012).
4. Hybridization. When genetically distinct populations interbreed, new alleles are introduced into the hybrid population. This can occur through migration, climate changes, or territorial expansion. Hybridization can lead to “adaptive introgression,” where the best-adapted hybrids form a new population with a mix of alleles from both parent populations, enhancing reproductive success (see, e.g., Racimo et al., 2015, 2017).
5. Recombination. Sexual reproduction scrambles DNA through crossover and recombination, ensuring that each offspring has a unique combination of traits (Stapley et al., 2017). During meiosis, chromosomes exchange DNA segments, and each egg or sperm receives a mix of parental DNA. This genetic diversity increases the chances that some offspring will survive environmental changes, as they are not all genetically identical. One caveat is that recombination is most beneficial under fluctuating or spatially variable selection and small populations (Otto & Lenormand, 2002; Stapley et al., 2017) and that temperature and condition alter recombination rates (Stapley et al., 2017).
6. Selection. Traits that enhance reproductive success are positively selected, while those that hinder it are negatively selected. Neutral traits have no effect on reproductive success. The optimal amount of a trait varies by environment; for example, dark skin is advantageous in sunny regions but disadvantageous in areas with little sunlight. Selection can also act on cultural traits, influencing allele frequencies over time (e.g., Tishkoff et al., 2007). Traits do not always become more complex; they can simplify, as seen in flightless birds or legless snakes.
Moreover, some additional mechanisms that collectively drive genetic variation and evolution are also worth mentioning, such as:
Turnover and aging. Faster generational turnover accelerates evolution by replacing older generations with new ones (see, e.g., Thomas et al., 2010). Aging is not biologically necessary, and species that live longer (e.g., Bristlecone Pine) evolve more slowly. The “Grandmother hypothesis” suggests that post-menopausal women increase their grandchildren’s survival, indirectly influencing selection (Herndon, 2010; Sear & Mace, 2008).
Environmental stability. In stable environments, populations reach equilibrium, reducing selection pressure and slowing evolution (Estes & Arnold, 2007; Orr, 1998). In changing environments, populations are further from equilibrium and evolve more rapidly. Strong selection pressure is evident when a new trait spreads quickly through a population (e.g., Kwiatkowski, 2005; Tishkoff et al., 2007).
Balancing selection. Although not cited in the book, this mechanism is particularly relevant for some genes, such as innate immunity genes. A study found that pathogen exposure has driven both positive and balancing selection in these genes, with balancing selection playing a major role in maintaining genetic diversity (Ferrer-Admetlla et al., 2008).
Chapter 5 – Selectors
A selector is anything that affects reproductive success by favoring certain traits. Early humans faced harsh selectors like climate, predators, and disease, which shaped the traits we have today. Modern humans, aided by science and technology, are less affected by these selectors.
Climate is the most powerful selector, affecting food availability and survival (Frost, 2019; Lynn, 2006). It includes temperature, precipitation, sunlight, and seasonal variations, which determine the type and quantity of food. Temperature, influenced by altitude and ocean currents, is a key factor, with sunlight being the primary driver. The equator receives more sunlight than the poles, creating significant temperature differences. Seasonal variations are most pronounced in temperate zones, where food is abundant in summer but scarce in winter. Seasonal changes affect food supplies, particularly in temperate zones where winters limit vegetation but allow large mammal hunting.
Catastrophic climate changes, such as ice ages and volcanic eruptions, have also shaped human evolution (Timmermann et al., 2022). The eruption of Mount Toba 73,000 years ago caused a volcanic winter, drastically reducing temperatures and sunlight, leading to widespread famine and population decline. This event particularly affected northern latitudes, where survival was already challenging, while Africa remained relatively unaffected.
Two major ice ages impacted human evolution: the first began 73,000 years ago, likely triggered by Toba (but see, Lane et al., 2013), and ended 55,000 years ago. Glaciers covered much of Eurasia, forcing populations south and creating conflicts. The Eurasian population declined sharply, and those better adapted to the cold survived, passing on cold-adaptive traits. As the climate warmed, glaciers melted, sea levels rose, and humans reoccupied Eurasia. The second ice age, between 30,000 and 12,000 years ago, was more severe but had less impact on physical evolution due to cultural advancements like clothing and shelters. Lower sea levels during this period allowed humans to migrate to new regions, such as North America and Australia. Despite population reductions, warming periods led to expansion, and agriculture (12,000 years ago) accelerated growth.
Sexual selection is another powerful selector. In K-strategy populations (fewer offspring, more care), individuals are more selective about mates, strengthening pair bonds and monogamy (Miller, 1994). Women, historically more dependent on men for resources, were often the primary selectors, choosing mates who could provide food and protection.
In Africa, women had greater selection influence because they gathered food. In colder climates, men had greater selection influence because they provided more food through hunting (Lynn, 2006). This led to Eurasian women becoming more beautiful due to male selection, while Eurasian men became harder workers and slightly more intelligent due to female selection. In contrast, African women may have become somewhat more intelligent than African men, who evolved to be more sexually attractive. African societies often prioritize a woman’s ability to work outdoors over physical beauty, leading to the evolution of traits that are less tied to survival.
Sexual selection in Africa has resulted in the evolution of “superfluous traits,” similar to how male birds develop bright plumage to attract mates. Women could afford to choose “cads” if it didn’t reduce their reproductive success. In Europe and Asia though, harsh conditions made it essential for women to choose resourceful partners. Men, who can impregnate multiple women, have a greater impact on future generations’ traits.
Men tend to select women based on health, fertility, and genetic quality, often indicated by physical symmetry and youthfulness. Women with lighter skin, associated with youth and beauty, are often preferred (Frost, 1990). This pattern holds true in all populations (Jablonski & Chaplin, 2000).
Humans, as group animals, evolved to cooperate because high status is crucial for reproductive success and expulsion from the group is feared due to its reproductive loss. This is why all individuals carry some “altruistic alleles” that code for behaviors that increase the group’s fitness, even at the expense of individual fitness (Davis et al., 2018; Henrich & Muthukrishna, 2021). Group animals experience social control emotions like guilt and shame, which enforce group norms. Sociopaths, who lack these emotions, are often excluded from groups. Cultural rules within groups can enhance competitiveness, and adherence to these rules is reinforced by social control emotions. Cold northern climates, where survival depended more on strict social norms, may have selected for stronger social control emotions than in the tropics.
Chapter 6 – Neoteny
Biologically, organisms reach sexual maturity when they can reproduce and physical maturity when they reach their adult form. These processes are controlled by different genes and can evolve independently. Populations can evolve to mature physically or sexually faster or slower, or both. Neoteny occurs when individuals retain juvenile traits into adulthood, either by accelerating sexual maturation while slowing physical development or by maintaining juvenile characteristics as they grow.
Humans are the most neotenous primates, often described as “sexually mature fetuses.” Neotenous traits include a flat face, less prominent jaw, smaller teeth, a larger skull relative to body size, less body hair, shorter limbs, and more subcutaneous fat—features common in primate infants (Bednarik, 2013). These traits, such as the centered occipital hole and forward-positioned vaginal opening, were crucial for human evolution. Neoteny also explains why humans have a white sclera, straight hair at birth, and lighter skin in newborns, which darkens with age.
Neotenous traits likely became advantageous as humans developed tools and weapons, reducing the need for robust jaws and large muscles. A larger brain, a neotenous trait, correlates modestly with intelligence (r=0.4; Gignac & Bates, 2017), which would have been beneficial as humans migrated to colder, seasonal climates requiring planning and problem-solving. However, larger brains are energy-intensive, consuming 20% of an adult’s energy and 75% of a baby’s.
In colder climates, neotenous traits like subcutaneous fat, flat faces, small hands and feet, and epicanthic folds (eye protection) helped conserve heat. East Asians are the most neotenous humans, with round heads, flat faces, and evenly distributed fat. Whites are slightly less neotenous, with longer limbs and less evenly distributed fat, while Africans are the least neotenous. One issue with the main assumption is that this “cold-adapted Mongoloid face” did not confer an advantage in maintaining warmer face temperature (So, 1980).
Chapter 7 – Genetic Distance
Reproductively isolated populations become genetically distinct over time due to different environmental selectors, a process known as the Founder Effect. Geographic separation, random mutations, and interbreeding with other populations contribute to genetic divergence. Genetic distance between individuals or populations is measured by comparing shared alleles, with identical twins having zero distance and unrelated individuals showing greater divergence. Based on several studies (Barreiro et al., 2008; Hawks et al., 2007), Fuerle argued that, over the past 60,000 years, genetic distances between races have increased due to faster evolution in different directions. Yet this claim is unsupported. Selection pressure may have accelerated greatly during the last 40,000 years (Hawks et al., 2007), yet at the same time large allele frequency shifts are shaped by neutral processes, especially population history, migration and drift (Coop et al., 2009). This suggests that mutations may be driven to intermediate frequency by strong selection, but subsequently drift to loss or fixation when the selective pressure weakens. Although some gene regions showed an increasing population differentiation, some others showed a reduced differentiation (Barreiro et al., 2008), as it probably depends on selection. For instance, relaxation of selection occurred on olfactory receptors because its role for survival and reproductive success has decreased (Pierron et al., 2013).
DNA sampling and SNP analysis reveal genetic distances between populations, ethnicities, and races. Studies from Cavalli-Sforza (1994, p. 82) show that Sub-Saharan Africans are the most genetically distinct from other populations, while Northern Eurasians and Southeast Asians are less related. East Africans, due to historical Eurasian migrations, are closer genetically to non-Africans than to other Africans.
Salter (2007, p. 64) calculated genetic distance calculations (Fst) between 26 populations (sampled from Cavalli-Sforza et al., 1994), and showed that Bantus and Australian Aborigines are the most genetically distant, while Bantus and San (South Africans) are closely related. A reproduction of the table is shown below:
There is even evidence, from a multivariate analysis based on genetic distances between populations, that human population structure inferred from the Y chromosome corresponds broadly to language families (Poloni et al., 1997). Other studies reported that populations that are adjacent in the genetic tree usually speak languages of the same family (Cavalli-Sforza, 2001; Henn et al., 2012b).
People tend to choose mates who resemble their opposite-sex parent, ensuring favorable traits are passed on (Bereczkei et al., 2004). This sexual imprinting effect has sometimes been replicated (Hou & Wang, 2021) and sometimes not (Heffernan et al., 2019; Marcinkowska, 2012; Zietsch et al., 2011). Studies show that individuals recognize relatives through facial or olfactory cues, and males prefer children who resemble them (Burch, 2021). Genetic similarity theory suggests that people are more likely to trust and help those who share their genetic traits, such as family members or individuals with similar appearances (Rushton & Bons, 2005; Rushton, 2009).
The concept of genetic distance has been misrepresented, with claims that humans are 99.9% identical across races. However, even small genetic differences (0.1% of 3 billion nucleotides) result in 3 million variations, and structural changes in DNA (duplications, deletions, inversions) can increase differences to 12%. For comparison, humans share 98.4% of genes with chimpanzees, 95% with dogs, and 74% with nematodes. The significance lies not in the percentage of shared genes but in which genes differ and how they are expressed.
Lewontin incorrectly claims that 85% of genetic diversity occurs within populations, not between them, suggesting racial classification is unnecessary. The argument ignores that most of this within-population variance was found to be within individuals, not between individuals (Fuerst, 2015; Sarich & Miele, 2004, pp. 166-169; see also, Chuck, 2011), that the common measure of genetic distance (Fst) underestimates population differences because the maximum Fst values are limited by heterozygosity (genetic diversity within populations), and that the average genetic variation across loci does not allow one to predict the amount of differentiation in loci that were under selection (Fuerst, 2015). Polygenic traits can diverge substantially even with high within-group diversity, as the evolutionary default model (assuming drift alone, no selection, despite unlikely) would predict a black-white IQ gap of d=0.75. Since the predicted gap is smaller than the observed gap of d=1.0, trait divergence exceeds neutral expectation and thus suggests directional selection with respect to IQ (Leinonen et al., 2013). Even when accepting at face value the 15% variation between populations, Wright considered that FST=0.15 reflects moderately great differences (Long, 2009). The original argument also ignores the importance of allele groups unique to each race. Genetic variation between populations differs qualitatively from genetic variation within a population, because genes that differ across population boundaries (e.g., geographic, ecological, or cultural divide) are genes that have higher selective value whereas genes that differ within a population have lower selective value (Frost, 2014b). Specific regions of the genome are indeed more differentiated across populations than others (Barreiro et al., 2008). And certain alleles are more common in some races than in others, and sometimes, much more so. These have been called “ancestry informative markers” (AIMs). The more AIMs that are being examined, the more accurately we can determine the person’s race (Sarich & Miele, 2004, p. 21). Unsurprisingly, the genetic relatedness measure, ω, can show clear distinctions between populations if enough polymorphic loci are used. How often are 2 people from one population more genetically different than 2 from different populations? With 10 loci: ~30%. 100 loci: 20%. 1,000 loci: ~10%. With many thousands of loci (and distant populations), the answer is never (Witherspoon et al., 2007).
Genetic similarity influences behavior, with people favoring those who share their traits, such as family members or individuals from the same ethnicity (Salter, 2007). Mixed marriages often compensate for ethnic differences by selecting partners with other similarities, such as educational attainment.
Chapter 8 – Evolutionary Psychology
Humans naturally prioritize their own children and relatives, practicing nepotism (Krupp et al., 2011) and showing greater generosity toward their own ethnicity (Salter, 2007, pp. 80, 102, 123, 146). Men, uncertain of paternity, often favor their sister’s children over their brother’s (Rushton, 1997, p. 75), as their brother’s wife might have been unfaithful (i.e., they cheated on their wife). Grandparents grieve more for their daughter’s children than their son’s children, because they are more certain they are related (Littlefield, 1986), i.e., their son’s wife may have cheated on him. And identical twins grieve more for their dead co-twin than do fraternal twins, who share fewer alleles (Rushton, 2005; Segal, 1999, pp. 176-178; Segal et al., 2002). This is why individuals help their mother’s sister’s children more than their other cousins (Jeon & Buss, 2007) and maternal grandparents are more willing to travel to see their grandchildren than paternal grandparents (Pollet et al., 2007, 2009) and granddaughters share greater intimacy with their maternal grandparents, especially maternal grandmothers (Tu et al., 2023). People assess relatedness through location (e.g., offspring in their nest) and traits (e.g., resemblance in appearance or behavior). Mothers often reassure fathers of paternity by highlighting a child’s resemblance to them, ensuring paternal investment. Unsurprisingly, we care more about our own children than the children of strangers, and children are much more likely to be abused by a parent if one of the parents is a stepparent (Daly & Wilson, 2001; Schnitzer & Ewigman, 2005; Tooley et al., 2006; Weekes-Shackelford & Shackelford, 2004; but see, Temrin et al., 2000).
Couples are happier when spouses share heritable traits, and biological siblings’ friends resemble each other more than adoptive siblings’ friends. People pick not only spouses (Bereczkei, 2008) and friends (Rushton, 1989) who have similar traits, and are therefore more genetically similar, but even pick pets that look similar to themselves. People prefer genetic similarity in social partners, and assort on the more heritable components of traits (Rushton, 2005; Rushton & Russell, 1985). Even in mixed marriages, couples compensate for ethnic differences by selecting partners with other similarities (Ahern et al., 1981). People also trust strangers more if their faces resemble their own (Krupp et al., 2008).
Historically, nations were built on genetic similarity to minimize conflict and promote shared interests. In multi-ethnic societies, prioritizing one group’s genetic interests can lead to ethnic conflicts, with severity tied to genetic distance between groups.
SECTION 2 – Traits of Living Populations
Fuerle argued that racial differences arise for the same reasons that different species do – populations become isolated and gradually change, and there is little inflow of alleles from other populations. Race is biologically real, goes beyond skin color, and is not a social construct (Coon, 1962, p. 662; Fuerst, 2015; Sesardic, 2010). Babies as young as 3 months prefer faces of their own race (Bar-Haim et al., 2006; Kelly et al., 2005), genetic analysis can identify self-reported race with great accuracy (Guo et al., 2014; Kirkegaard, 2021; Tang et al., 2005), machine learning can identify self-reported race with accuracy (Gichoya et al., 2022) as well as its relationship with intelligence (Kirkegaard & Fuerst, 2023).
Chapter 9 – Hard Tissue
As a result of evolutionary adaptations, skull morphology varies substantially among races. Asian skulls are round with flat faces, while Caucasian skulls are longer but share similarities, suggesting recent divergence or interbreeding. A simple test for Asian skulls is placing them face down; if they rest on cheekbones without tipping, they are likely Asian. African skulls differ markedly, being narrower with denser, thicker bones. They feature rounder, larger eye sockets, wider nasal openings, and pronounced prognathism (protruding jaws), traits shared with ancient human fossils and apes. African skulls also exhibit a sagittal keel (a ridge along the skull) and post-orbital constriction, indicating strong jaw muscles and a smaller forebrain (the center of planning and abstract thought).
At birth, African infants have fewer cranial bones than Eurasians, with thicker, denser bones that provide durability but reduce swimming ability. Eurasians have thinner, lighter (gracile) bones, while Africans have robust bones. The cephalic index (skull width/length ratio) is highest in Asians and lowest in Africans, reflecting heat retention adaptations. Africans have long, narrow skulls (dolichocephalic) and lose heat the fastest, while Asians have rounder skulls (brachycephalic) and retain heat better.
The occipital bun, a bulge at the back of the skull, is more common in Africans and Neanderthals, suggesting a primitive trait. African skulls may also feature a post-bregmatic depression (a bump at the skull’s top), another primitive characteristic. The foramen magnum (spinal cord opening) is positioned differently across species: vertical in humans, oblique in apes, and horizontal in monkeys, reflecting bipedalism.
Supraorbital ridges (bony ridges above the eyes) are prominent in populations with large jaws and teeth, such as herbivores or early humans. These ridges diminished as cooking reduced the need for powerful jaws. Eye socket shapes vary: East Asians have round sockets, Australian Aborigines have rectangular ones, Africans and Europeans have square or rectangular sockets, Europeans exhibit more slanted eye sockets, and Neanderthals have round ones.
The nasal prominence (nose bone projection) is highest in Europeans and least in Africans, whose nasal bones are flat. The nasal index (width/height ratio of the nasal cavity) is highest in Africans and lowest in Caucasians. The anterior nasal spine, supporting a protruding nose, is prominent in Caucasians, less so in Asians, and absent in Africans. This feature can determine race: a pen placed through the nasal cavity stays in Caucasians but rolls in Africans.
Simian prognathism (protruding jaw) is a primitive trait seen in monkeys and some African skulls, useful for fighting and biting. The facial angle (forehead-to-jaw inclination) is lower in Africans (70°), resembling early humans like Homo habilis and erectus, while modern humans have higher angles (up to 100°), reflecting a larger prefrontal cortex and advanced cognitive functions. This angle correlates with brain development, particularly in the frontal lobe, responsible for planning and self-control.
The absence of prognathism (protruding jaw) is associated with higher intelligence and a more modern appearance, as it allows for a larger brain (Lieberman, 2011, p. 173). A chin strengthens the jaw, particularly in gracile (modern) jaws, preventing fractures. Only Homo sapiens sapiens (Hss) fossils have chins, though not all modern humans exhibit prominent chins. The jaw can be reinforced by thickness, a chin, or a Simian Shelf (a bony protrusion behind the lower incisors), found in apes and Neanderthals but absent in most modern humans (Coon, 1962, p. 349).
The sacral index (width/length ratio of the sacrum) reflects evolutionary adaptations to bipedalism, with higher indices indicating better support for internal organs. Africans have the lowest sacral index, while Europeans and others, including Australian Aborigines, have higher indices due to shared ancestry with archaic humans from Western Asia.
Limb proportions, measured by the brachial index (radius/humerus), crural index (tibia/femur), and humero-femoral index (arm/leg length), indicate adaptations to movement. Africans have limb proportions closer to apes, reflecting adaptations to warmer climates (Allen’s rule), while shorter limbs are selected for in colder climates.
Hand and finger proportions also differ by race. The 2D:4D ratio (index to ring finger length) is influenced by prenatal hormone exposure, with lower ratios (longer ring fingers) linked to higher testosterone (Manning et al., 2007; Manning et al., 2021; Manning & Fink, 2021; Xu & Zheng, 2015; but see Beaton et al., 2011; Richards et al., 2022) and better numerical skills. Africans, who have lower 2D:4D ratios, based on self-measured ratio (Manning et al., 2004, 2007) and radiographic digit ratios (Trivers et al., 2020), also have higher testosterone and fertility levels, consistent with the association between higher fertility and lower digit ratios (Brosnan, 2006). While the 2D:4D ratio correlates with several psychological and health outcomes (Manning & Fink, 2021), there are several unresolved issues, such as why androgen receptor variants are not associated with digit ratios (Voracek, 2014), and why this measure does not predict criminality (Pratt et al., 2016), although explanations and solutions have been provided in a review (Manning & Fink, 2021).
African heel bones project more, providing advantages in sprinting and jumping, which explains West African dominance in such sports. African feet are flatter, with greater separation between the first and second toes, further reflecting adaptations to their environment and physical demands.
Chapter 10 – Soft Tissue
Brain size and structure: East Asians have larger brains and more neurons than sub-Saharan Africans, with a higher brain-to-body mass ratio. Brain size heritability is estimated at 0.9, and a correlation of 0.41 exists between brain size and IQ. Some updated figures are worth reporting: 1) brain measures and regions are about 60-80% heritable twin studies (Jansen et al., 2015) and regional brain volumes have a median SNP-heritability of 34.8% (Zhao et al., 2019), 2) a meta-analysis reported a mean correlation of 0.31 between brain volume and IQ, that was moderated by test quality, with correlations of 0.23 and 0.39 for fair and excellent tests, respectively (Gignac & Bates, 2017). The brain’s surface folds (gyri and sulci) increase cortical area, with more complex brains having more folds. Africans and some individuals with cognitive impairments have fewer convolutions, and Aboriginal Australians have smaller, less complex brains (Baker, 1974, p. 293). The supragranular layer of the cerebral cortex is 15% thinner in Blacks than in Whites. The prefrontal region, responsible for planning and abstract thought, is larger in humans (29%) than in chimpanzees (16.9%) or cats (3.4%). Africans have more developed rear brains (vision) and less developed frontal brains (planning), as reflected in their skull shape.
Sexual dimorphism and organs: Larger testes in Africans suggest higher promiscuity (although the reference provided by Fuerle showed ambiguous findings), as males with larger testes produce more sperm (Lüpold et al., 2020), increasing reproductive success. Africans also have larger sexual organs (see also, Alves Barboza et al., 2018; Francis & Kirkegaard, 2021). Apocrine glands, which produce body odor, vary by race (Baker, 1974, pp. 173-176; Coon, 1962, p. 116), with Africans having more chloride in sweat and Asians having less body odor. Odor plays a role in identifying genetic similarity and mating receptivity.
Sclera and social adaptation: Humans have a white sclera, unlike other primates, aiding communication by revealing gaze direction (Bettle & Rosati, 2021; Lieberman, 2011, p. 388; Tomasello et al., 2007). Some Africans and Australian Aborigines have slightly yellowish sclera, possibly due to melanin. High intelligence and social complexity are linked, with consciousness potentially evolving as a social adaptation.
Facial and nasal traits: Everted lips in Africans may signal health or serve as a cooling mechanism. Narrow noses, common in colder climates, warm and moisten air, while wide noses are adaptive in warm, humid environments (Bastir et al., 2024; Coon, 1962, p. 62; Doddi & Eccles, 2011; Maddux et al., 2017; Zaidi et al., 2017). Neanderthals had large nasal openings, possibly for sudden energy needs. Nasal indices (width/length ratio) reflect evolutionary adaptations, with early hominids having wide noses near the equator.
Body fat and muscle: Subcutaneous fat retains heat, beneficial for babies. Africans have less body fat and more muscle than Whites, with fat concentrated in the buttocks to lower the center of gravity and aid movement.
Skin color and hair: Skin color evolves based on UV exposure, with melanin protecting against harmful rays (Jablonski & Chaplin, 2000). Lighter-skinned Whites are more prone to skin cancer than darker-skinned Whites, while Blacks are more resistant to skin diseases. Asians have more subcutaneous fat (Wells, 2016, pp. 116, 119, 123, 182), which might explain their yellowish tint. Skin color heritability is obviously heritable. Curly hair in tropical regions aids sweat evaporation and brain cooling. African hair grows slowly and is fragile, while Asian hair grows fastest and is most elastic. Africans have the shortest hair, Asians the longest. Baldness is more common in White and African males than in Asians. Some Africans, especially females, have receded hairlines, a trait shared with bonobos and orangutans.
Muscle fibers and athletic performance: Type 1 (red) muscle fibers provide endurance, while Type 2 (white) fibers offer power. East Africans, with more red fibers, excel in marathons, while West Africans, with more white fibers, dominate sprinting and jumping (Ama et al., 1986; Fuku et al., 2019). Some studies showed that the proportion of type I fibers is higher in Caucasian populations than in those of African ancestry (Fuku et al., 2019). Eurasians, with fewer fast-twitch fibers, likely evolved greater intelligence at the expense of athletic ability, relying more on tools and weapons.
Hormones and behavior: Higher testosterone levels correlate with earlier sexual maturity, higher fertility, and increased crime rates (Armstrong et al., 2022). Testosterone declines in monogamous males, indicating reproductive success. Low serotonin levels, linked to impulsivity and violence, are more common in Africans. Studies controlling for socioeconomic status (SES) often obscure racial differences, as SES and IQ are correlated with race.
Race and health: Race is not a social construct (Fuerst, 2015). Moreover, racial differences in reactions to chemicals and biological substances exist. Blacks have rare blood types (e.g., Hodgson et al., 2014b) and are more prone to prostate cancer (Rebbeck, 2018), tuberculosis (Cantwell et al., 1998), and sickle cell anemia (Solovieff et al., 2011), while Europeans are more sensitive to cystic fibrosis (O’Sullivan & Freedman, 2009), and Jews are affected by Tay-Sachs disease. Racial differences in red blood cells, hemoglobin (Beutler & West, 2005), lung capacity (Guidot et al., 2025; Whitrow & Harding, 2008), and body composition (Wagner & Heyward, 2000) are also documented. In 2005, the FDA approved BiDil™, a drug effective for African Americans but less so for Eurasians, highlighting racial disparities in disease susceptibility and treatment (Temple & Stockbridge, 2007). Indeed, ignoring admixture in an individual with admixed ancestry will complicate the promise of personalized medicine (Mersha & Abebe, 2015).
Chapter 11 – Reproductive Strategy
Living beings use two reproductive strategies: the r strategy (producing many offspring with little investment, ensuring some survive) and the K strategy (producing fewer offspring with more investment, increasing each one’s survival chances). For example, salmon use the r strategy, while elephants use the K strategy.
Cranial sutures in Africans close earlier than in Eurasians, indicating less neoteny (e.g., Schwartz, 2005, p. 131) and shorter brain growth periods. Brain size differences are evident at birth and even in the fetal stage. Multiple sources suggest that African children’s intellectual maturation peaks earlier, but IQ disparities with Eurasians widen with age (for possible contrary evidence on the IQ gap, see Malloy, 2013).
Black women have three times more premature births than White women (Rushton, 1997, pp. 125, 252, 267), and disparities persist even after adjusting for social status (Manuck, 2017), and shorter gestation periods (Rushton, 1997, pp. 147-148). Their childbirth complications are fewer due to smaller, elongated fetal skulls. African fertility rates are extremely high, with African women having far more descendants than White women. Sub-Saharan Africa has 40 births and 16 deaths per 1,000 individuals annually, driven by high fertility rates and aided by subsidies.
Chapter 12 – Behavior
The argument has been made by Damasio (1994) that without inherited emotions that motivate at least some behavior, a living thing would have no motivation to do anything. Behavioral traits such as personality, parenting, delinquency, psychiatric disorders are under genetic influence. Even traits such as facial expressions (Peleg et al., 2006), circadian preferences (Hur et al., 1998; Toomey et al., 2015), humor (Baughman et al., 2012; Cherkas et al., 2000; Vernon et al., 2008), loneliness (Gao et al., 2017), physical activity (den Hoed et al., 2013; Gielen et al., 2014), are heritable. One important trait though is the ability to delay gratification. This characteristic varies by race, being highest in Asians and lowest in Africans, who more often prioritize immediate rewards over long-term planning. This tendency is linked to environmental factors, such as the abundance of food in tropical regions, which historically reduced the need for Africans to develop patience or self-control. In contrast, colder climates forced Eurasians to store food, fostering planning and delayed gratification. Evolutionary pressures shaped many other important behavioral outcomes. And some are worth mentioning.
Reproductive strategies: Promiscuity correlates with larger testicles, as males producing more sperm have a higher chance of fertilizing eggs. However, this trade-off reduces investment in brain development, as resources are diverted to reproduction rather than cognition. Monogamy, associated with larger brains (Dunbar, 2009; Schultz & Dunbar, 2007) and paternal care (Lukas & Clutton-Brock, 2013), is more common in colder climates, where men must provide for their families. In contrast, polygamy and unstable pair bonds prevail in warmer climates, where women gather food independently.
Pair bonding and evolution: Monogamy was reinforced by concealed ovulation in women, encouraging men to stay close to ensure paternity (Rodriguez-Girones & Enquist, 2001) and benefitting females by allowing them to avoid aggression from other females (Krems et al., 2021). This pair bonding was crucial for human evolution (Chapais, 2008), showed association with brain size (Schillaci, 2006), but likely originated outside Africa (and thusly undermining OoA), as African societies exhibit less stable pair bonds. The hormone oxytocin fosters feelings of love and, as a result, strengthens pair bonds by reducing interest in alternative partners (Gonzaga et al., 2008).
Sexual behavior and anatomy: Africans exhibit higher promiscuity, reflected in larger genitalia and higher rates of sexually transmitted diseases. Their sexual anatomy and behaviors suggest possible front-to-back mating, as indicated by the large fleshy rumps of some populations, e.g., Andaman Islanders, Hottentots, and Bushmen or by the popularity of anal homosexuality among African American men. Perhaps the most striking feature is that “While the female genitals in Orientals are “front and high,” in Africans they are “back and low”; erections in Orientals are “parallel to the body and stiff” but in blacks are “at right angles to body and flexible,” which also suggests front-to-back mating.” (see, Rushton & Bogaert, 1987).
Slavery and cannibalism: This has been practiced by all races throughout history, but today, only Africans openly engage in it. In the U.S. prior to 1865, some black slaves who had been freed even purchased their own black slaves. If whites hadn’t bought slaves, many Africans would have been killed or eaten, as slaves had no value unless sold (Baker, 1974, pp. 364-365, 391). Cannibalism (still practiced in Africa) reflects a lack of empathy and is linked to psychopathy. Such behaviors are reduced in colder climates, where cooperation and trust are necessary for survival.
Crime and psychopathy: Criminal tendencies are heritable, with blacks and Native Americans scoring highest on psychopathy tests, followed by Hispanics, whites, and Asians (Lynn, 2019). Blacks commit significantly more crimes than whites, with black-on-white crimes far exceeding white-on-black crimes. Black crime is often more impulsive and violent, involving multiple offenders.
Spousal homicides: Black spousal homicide rates are many times higher than whites’, driven by anger and status concerns. Interracial marriages, particularly between black men and white women, are less stable and more prone to violence (Mercy, 1989). White women in such marriages face higher risks of being killed by their black husbands, as rejection threatens the husband’s social status.
Rape and reproductive strategies: Africans have high rape rates globally, likely due to historical self-sufficiency of African women. Thus, rape is likely to result in living children. In colder climates however, rape becomes maladaptive; pair bonding increases reproductive success, as men who supported their families had better outcomes.
Chapter 13 – Genes
Humans share the same genes, but the frequency of specific alleles varies across populations, influencing traits like brain development, skin color, and behavior. Key genes include:
DAB1: Involved in cerebral cortex organization, a specific allele has become universal in Chinese but not in other populations.
MCPH1 (microcephalin): Affects brain size and intelligence (Woodley et al., 2014). A variant that arose 37,000 years ago is common in Eurasians but rare in Africans and absent in chimpanzees.
DRD4: Controls sex drive and has been linked to novelty-seeking traits in Europeans, though findings are inconsistent.
AVPR1a: Influences social bonding and altruism, with longer RS3 alleles associated with greater altruism.
SLC24A5: Determines lighter skin in Eurasians, with different alleles in Europeans and Asians. This gene is also active in the brain.
PDE4: Linked to lung cancer susceptibility, with blacks being more vulnerable than whites, possibly due to less historical exposure to smoke in tropical climates.
Chapter 14 – Intelligence
Around 30,000 years ago, human brain size began to decrease despite longer lifespans, possibly due to the survival of less intelligent individuals enabled by abstract thinking and agriculture. Mutations around 2 million years ago likely made brains more efficient, allowing smaller brains to achieve similar intelligence. Yet intelligent brains are still more efficient, and genes associated with human brain evolution (HAR-BRAIN genes) are more expressed in areas related to higher-order cognitive processing (Wei et al., 2019). As expected, IQ is highly heritable, with strong correlations between spouses’ IQs (0.4) and educational backgrounds (0.6). Importantly, national IQ correlates with GDP per capita (Lynn & Becker, 2019), and analyses based on national IQ are still robust.
Brain regions like the Broca and Wernicke (language) areas, crucial for abstract reasoning, show high heritability (Thompson et al., 2001), as is the case of other brain regions (Jansen et al., 2015; Zhao et al., 2019). IQ remains stable over a lifetime, with childhood IQ strongly predicting adult IQ (Breit et al., 2024; Deary et al., 2000; Deary, 2014). Men’s intellectual maturation takes longer, leading to higher IQ scores in adulthood compared to women (Parra & Kirkegaard, 2025), although not necessarily latent scores (Hu, 2025).
IQ levels set the limit to what is possible and not possible: Africa, despite its resource wealth, has never developed a civilization, while resource-poor East Asia has experienced rapid economic growth. Egalitarians are eager to close the mysterious Black-White IQ gap, and expensive intervention programs were designed with the sole purpose of sustainably boosting IQ among disadvantaged children (often blacks). All have failed. IQ heritability is high and does not vary by race (Pesta et al., 2020). The Flynn effect, often cited to challenge IQ heritability, does not reflect an increase in intelligence. If intelligence level is truly changing, then a decline is more likely, as indicated by grade inflation, dumbing down of SAT, courses, culture, etc.
The existence of racial differences in “organic” retardation suggests there are genotypic IQ differences. Whites with IQs around or below 70 typically suffer from genetic anomalies, while blacks with similar IQs function normally. For whites, an IQ of 70 is ‘pathological’, while for blacks, an IQ of 70 is ‘normal’. The environmental theories failed to account for the racial gaps (Dalliard, 2014; Hu, 2014; Jensen, 1973, 1998; Rushton & Jensen, 2010). Racial IQ gaps persist despite reduced racism (Fuerst, 2013; Murray, 2021), increase with education levels (Hu, 2023, 2024), and stereotype threat effects are artefacts unrelated to intelligence and group differences.
There is a highly positive correlation between distance from the equator and IQ, a highly negative correlation between IQ and low winter temperature (Templer & Arikawa, 2006; Templer & Stephens, 2014). These findings are consistent with the high correlation between cranial capacity and distance from the equator (Beals et al., 1984). Some outliers are worth noting (Lynn, 2006). For instance, the lower IQ of the Inuit (91) suggests fewer selectors for high intelligence in stable Arctic environments compared to temperate regions. In the Arctic, reliance on sea animals year-round reduces the need for complex skills or food storage, limiting the demand for high intelligence. Fuerle argued that “A highly seasonal climate is more mentally challenging because of the many additional problems that must be solved in order to survive.” while Lynn (2006) argued that “The Arctic Peoples were subject to extreme cold stress but comprised very small populations, so they would be unlikely to have had mutations for higher intelligence.”
Humans already invested a lot of resources in the brain (about 25% of human metabolism supports brain function), and further brain growth would likely strain other organs (Dunbar, 1998) but likely due to energy trade-offs with other tissues such as adipose tissue (Navarrete et al., 2011), resulting in lower reproductive success (Isler & van Schaik, 2006). Optimal brain size balances intelligence with reproductive success, as excessive brain size can hinder survival. Investing more resources in a larger brain implies fewer resources devoted to muscles, bones, and speed necessary for either hunting or escaping other animals. Optimal intelligence depends on environmental demands but also on other traits that the individual possesses. And in Africa, optimal intelligence was lower due to fewer selective pressures (Lynn, 2006). Habitats within Africa are indeed less diverse and complex than outside (Huang, 2025b).
That Northeast Asians have an average IQ of 105, while Southeast Asians average 87, may indicate that intelligence became less needed for reproductive success in the tropics. A mutation around 50,000 years ago, marking the “human revolution”, may have increased brain efficiency and thus allowed higher intelligence with smaller brains. The shift from hunting to agriculture reduced selection pressures for intelligence, as farming required less optimal levels of intelligence and brain size than hunting. Somewhere between 100,000 and 35,000 years ago, the human brain gradually became more globular and, as a result, the features of behavioral modernity accumulated gradually with time (Neubauer et al., 2018). Most studies and reports however suggest that the reduction in brain size occurred much later, i.e., during the Holocene (Cochran & Harpending, 2009, p. 112; DeSilva et al., 2023).
Agriculture and population growth initially reduced the need for high intelligence, but once populations expanded to the greater carrying capacity made possible by agriculture and private property made brains pay off again, higher intelligence became advantageous again. Predators generally have higher intelligence than non-predators, and domesticated animals have smaller brains and lower intelligence than their wild counterparts (Coon, 1962, p. 117), suggesting domestication favors docility and neoteny (Bednarik, 2013). Similarly, agriculture may have selected for neoteny in humans, making them less wild and more aesthetically pleasing.
Chapter 15 – Civilizations and Achievements
The major anatomical difference between archaic man (sapiens) and modern man (sapiens sapiens) is that the latter was more gracile; fewer resources in the organism were spent on bones and muscles, especially in the Holocene (Cartwright et al., 2024). Skeleton gracilization (i.e., lower bone mass and strength relative to body size) is likely driven by genetic factors like LRP5 variants which are known to regulate bone mass (Roca-Ayats et al., 2024). Gracility is the consequence of a shift in subsistence pattern from hunting-gathering to agriculture, which led to reductions in overall physical activity levels (Ryan & Shaw, 2015), or reduced bite force per chew due to tool use (Lieberman, 2011, pp. 272-279; Zink & Lieberman, 2016), or relaxing of selective pressures that maintain features that favor forceful biting (Katz et al., 2017; Ledogar et al., 2016). Before the human revolution 40-50,000 years ago, which marked the appearance of modern human behavior and cultural innovation, human progress was painfully slow (Cochran & Harpending, 2009, pp. 32, 64, 225-226; Corballis, 1993, p. 64). After the human revolution, a transition that was likely gradual (McBrearty & Brooks, 2000), men designed better tools and no longer let the bodies (dead) rot or be eaten by animals. They buried the dead, often with valuable objects, because their mind could imagine life after death. Drawings and sculptures also showed evidence of abstract thinking.
What happened? Fuerle argues this could be due to a newly discovered allele of the MCPH1 (microcephalin) gene which appeared about 37,000 years ago. This allele, along with another similar allele of the ASPM gene, which appeared even more recently, is still rare among Africans. This allele affects brain size and intelligence and may explain some of the differences in IQ between Eurasians and Africans and their capacities for civilized behavior, although multiple research denied such a relationship (Bates et al., 2008; Dediu, 2021; Dobson-Stone et al., 2007; Rushton et al., 2007) while others validated it (Frost, 2008; Rindermann, 2007; Woodley et al., 2014).
Civilization is a good indicator of the level of intelligence of a people and its evolution from archaic man. The size of civilization is compatible with the traits they possess, notably complexity and brain size. Baker (1974, pp. 507-508) argued that a society gives birth to a civilization (having features by which societies, commonly regarded as civilized in the everyday sense of the word, are distinguished from others to which this term is not ordinarily applied) if, prior to influence from outsiders, most of its members met most of the following requirements:
1. They cover themselves with clothes.
2. They keep the body clean and take care to dispose of its waste products.
3. They do not practise mutilation of the body, except for medical reasons.
4. They have knowledge of building in brick or stone, if those are available.
5. Many live in towns or cities, linked by roads.
6. They cultivate food-plants.
7. They domesticate animals and use some of the larger ones for transport.
8. They have knowledge of the use of metals, when available.
9. They use wheels.
10. They exchange property by using money.
11. They order their society by a system of laws.
12. They permit accused persons to defend themselves.
13. They do not use torture to extract information or for punishment.
14. They do not practise cannibalism.
15. Their religious systems include ethical elements and are not superstitious.
16. They use a script (not pictures) to communicate ideas.
17. There is some facility in the abstract use of numbers.
18. A calendar is in use, accurate to within a few days in the year.
19. Arrangements are made for the instruction of the young in intellectual subjects.
20. There is some appreciation of the fine arts.
21. Knowledge and understanding are valued as ends in themselves.
Based on several accounts (see, e.g., Baker, 1974, pp. 357-360, 368-373, 375, 394-396, 408-409, 520-521), Fuerle as well as Baker observed that Africans failed to meet most of these requirements. Baker even concluded that “The only race other than the Europid and Mongolid in which any close approximation to civilization ever originated is the Indianid.” Of note though, some African tribes had a calendar (Peek & Yankah, 2004).
The Great Zimbabwe is the largest ruin in sub-Saharan Africa yet the Africans probably served as laborers. Fuerle does not believe that they directed its construction because it is not representative of the culture and architectural tradition of Zimbabwe and Africans do not use stone construction. The case of Great Zimbabwe is complicated though; it is possible that the work can be attributed to the Shona tribe (Peek & Yankah, 2004, p. 874), despite a sharp change in style at a later time, and that the building in hewn stone was likely influenced by contact made with foreign traders (Baker, 1974, pp. 405-408).
When the whites were expelled from South Africa in 1994, GDP immediately experienced a sharp decline. In fact, the exodus of whites (especially the highly educated ones) had already begun shortly before this date, and GDP growth was already showing signs of exhaustion. The average income of all its inhabitants dropped by 40% between 1995 and 2000, and the income gap between whites and blacks narrowed until 1994. After that date, the gap increased, as did the number of South Africans living on less than $1 per day. One comprehensive account of post-apartheid South Africa suggests everything went awry after the exodus of whites (Braun, 1998a, 1998b). And other places as well. Rhodesia, as it was called when it was ruled by whites, was the breadbasket of Africa. Zimbabwe, as it was renamed after Africans took over, didn’t even manage to feed half of its population. Even Liberia, founded by repatriated American slaves, is dissolving into chaos and cannibalism, despite the infusion of African Americans who had lived in a white country. Thus it seems that blacks are unable to maintain a civilization when left to themselves. All examples testify to this. One stellar example was the luxurious Grande Hotel in Beira, built in the 1950s, which became a few decades later home to a community of squatters.
Charles Murray (2003) catalogued man’s accomplishments according to the number of times they were cited by others. According to this, 97% of the most important scientists and 74% of the most important artists and authors were white. Almost all men, and mostly from 4 countries: England, Germany, France, Italy. The rest were mostly Asians. But no blacks. The inventions purportedly made by blacks have been largely exaggerated.
Large mammals disappeared from Australia 45,000 years ago, from Europe and northern Asia 10,000 years ago, and from North America about 11,000 years ago; and, interestingly, all of this coincides with the arrival of Eurasians. In contrast, large mammals in Africa (elephants, giraffes, rhinoceroses, hippopotamuses, lions, gorillas…) still live today. If Africans have no qualms about killing these animals, a fair conclusion would be that the animals have not yet been killed because the Africans had not invented the means to do so.
Chapter 16 – Primitive Traits
If hairiness is a primitive trait, it is puzzling that Africans are less hairy than Caucasians. The explanation would be that hair reduces the effectiveness of body cooling through sweating. The loss of body hair in humans was accompanied by enhanced barrier functions of the stratum corneum, including the evolution of other epidermal keratins, which reduced the skin’s permeability and improved its abilities to resist abrasion and microbial attack (Jablonski & Chaplin, 2010). Hair is adapted for early monkeys and becomes maladapted for bipedal monkeys. Hair is suited for men in cold climates, before becoming maladapted when they had clothing.
Primitive traits, often linked to tropical origins, are retained in descendants because they may serve new purposes. For example, long arms, useful for tree-swinging in ancestors, now aid heat dissipation and throwing. Not all tropical traits are primitive; some, like disease resistances (e.g., sickle cell anemia), evolved uniquely in tropical humans (Piel et al., 2010).
Sexual dimorphism has declined from australopithecines to modern humans, influenced by sexual selection. Selecting mates for their masculinity and femininity increases sexual dimorphism and selecting mates who will pair bond reduces it. Thus, monogamous social systems tend to have lower dimorphism (Plavcan, 2018), and Asians are the least sexually dimorphic of the major races. Technological advancements, like cooking and improved weapons, have reduced the need for robust, primitive features (e.g., large teeth, heavy skulls, stronger muscles), leading to gracile traits (Ledogar et al., 2016; Zink & Lieberman, 2016) and possibly and larger brains.
Primitive traits, such as large jaws and heavy bones, are perceived as threatening but also signify masculinity. Dark skin, selected in the tropics, is costly but necessary for UV protection, while lighter skin in temperate zones avoids vitamin D deficiency. Africans are born lighter, but darkening after birth, with palms and soles remaining white. Other primitive traits, resulting from evolutionary adaptations and sexual selection pressures, include yellowish sclera (seen in gorillas, some Africans, and Aborigines), a higher larynx in monkeys limiting vocal range and sound control, and large mouths for biting, but also flat noses and protruding buttocks which are traits typical of female primates.
SECTION 3 – The Out-of-Africa Theory
A proposed model for the diversification of modern human lineages (taken from Cole et al., 2020) looks like this:
Afrocentrists believe that African erectus evolved into Homo Sapiens (Hs), then into Homo Sapiens Sapiens (Hss), and later migrated out of Africa, replacing the primitive peoples living in Europe (Neanderthals) and Asia (Homo erectus). Once modern Africans moved into Eurasia, they are thought to have lost all African traits, which were replaced by Eurasian traits.
On the other hand, since OoA holds that Hss, modern humans, arose 160,000 ya in Africa, the migration of Hss out of Africa must have occurred tens of thousands of years after that (Lewin, 1993, p. 98), which raises the question of what took them so long to leave? A possible scenario is that humans (or close relatives) left Africa way earlier than the postulated OoA migration 60-70,000 years ago. Indeed, the finding that Late Pleistocene Neanderthal mtDNAs originated through gene flow from Africa around 270 kya (Posth et al., 2017) contradicts the OoA model which predicts that humans only left Africa after evolving into modern form but is more consistent with the Out of East Asia model which predicts that the first split among major human groups occurred 2 million years ago (Zhang & Huang, 2019).
A question worth asking is: Why did African erectus become sapiens, but not Asian or European erectus, even though the Eurasian environment was more selective in evolving primitive traits into modern traits? The answer from OoA would be that the evolution into Homo Sapiens Sapiens was a random event: “Africa just got lucky.” However, this luck is overstated.
Chapter 17 – Fossil Skulls
The multiregional theory suggests modern humans evolved both inside and outside Africa, as independent evolution is common in nature (e.g., flight in insects, birds, and mammals). Fuerle argued that if modernity requires changes in multiple genes, parallel evolution is unlikely, but if it hinges on a single gene such as a Hox gene that turns a host of other genes on or off, it becomes plausible.
These African skulls were cited as supporting OoA:
Herto Skulls (160,000-154,000 years old): Classified as Homo sapiens idaltu, these skulls show primitive traits like large orbits, brow ridges, and a receding forehead. Their cranial capacity (1450 cc) is higher than modern Africans (1338 cc) but lower than Asians (1491 cc), suggesting brain size shrank in Africa. Herto’s location near the Middle East implies possible hybridization between Eurasian Homo sapiens and African Homo erectus.
Omo Skulls (195,000 years old): Just like Herto, the Omo skulls were found near a river in Ethiopia. Only two fossils were recovered and they both exhibited several primitive features (Fleagle et al., 2008), and therefore are likely not modern. Its proximity to the Middle East supports the idea of Eurasian influence.
Kabwe (300,000-125,000 years old): Found in Zambia, this skull has a cranial capacity of 1280-1300 cc, with prominent brow ridges, sloping (i.e., receding) forehead, and a sagittal keel. This skull looks much more primitive than the two others, perhaps because Eurasian migrants (from the Middle East) would not easily reach its location (deep in the interior of southern Africa).
Some unearthed Asian Skulls provide possible counterweights:
Peking Man (500,000-300,000 years old): It shows primitive traits like brow ridges and a sagittal crest and has been classified as an Erectus, but it has modern teeth and tools, indicating advanced cognition.
Dali (209,000 years old): It has a mixture of erectine traits (sagittal keel, heavy brow ridges) and sapiens traits (delicate cheek bones, flat face) (Athreya & Wu, 2017).
Jinniushan (260,000 years old): Classified as Homo sapiens, this female skull has a cranial capacity of 1330 cc, suggesting advanced evolution in Asia. Although this is comparable to the average of today’s Africans, it should be noted that women have smaller skulls than men.
Liujiang (150,000-87,000 years old): It is definitively a modern human skull with refined features, proving modern humans existed in Asia before the supposed OoA migration 65,000 years ago. Some recent estimates however suggested that its age range was much more recent, i.e., 33,000-23,000 years ago (Ge et al., 2024).
Asian skulls share shovel-shaped incisors, absent in Africans, and show continuity in dental patterns, supporting independent Eurasian evolution. Evidence of fire and tools in Asia dates back 500,000-800,000 years, earlier than in Africa. This last point though is unsupported as fire and tools existed in Africa even prior to these dates (Gowlett, 2016).
Homo floresiensis (18,000 years old): This “Hobbit” skull (417 cc) from Flores, Indonesia, shows primitive traits like a protruding jaw and large teeth. Its existence contradicts OoA, as it implies either rapid evolution or that modern humans coexisted with non-modern humans. But recent estimates suggested that its age range was much older, i.e., 100,000-60,000 years ago (Sutikna et al., 2016).
Other fossils from China, such as the Yunxian skull from Hubei, dated to 1 million years, the Hualongdong skull HLD6 from Anhui, dated to 300,000 years, the Dingcun teeth from Shanxi, dated to 270,000 years, a massive cranium from Harbin, dated to 146,000 years, all exhibit many modern traits (Huang, 2025a; Liu & Wu, 2022; Ni et al., 2021).
Chapter 18 – Modern Behavior
Paleoanthropologists link modern anatomy to modern behavior, such as tool-making, art, and burial practices. If Omo skulls are modern, as Afrocentrists claim, why didn’t Africans exhibit modern behavior earlier? Modern behavior requires social organization, which depends on environmental conditions favoring complex social relationships. Contra Fuerle’s view though, there is some evidence of modern behaviour, as illustrated by ornaments in the African Middle Stone Age (McBrearty & Brooks, 2000) and intentional images from the Blombos Cave and Diepkloof Rock Shelter engravings (Henshilwood et al., 2002; Tylén et al., 2020) and archaeological discoveries in the Border Cave (d’Errico et al., 2012).
Africans’ inability to reach nearby islands like the Canaries, Zanzibar, and Madagascar (only after the Indonesians), despite their proximity, contrasts with Asian Homo erectus, who crossed waters to Flores 800,000-900,000 years ago (Morwood et al., 1998; O’Sullivan et al., 2001). This raises questions about Africans’ capacity for modern behavior, such as raft-building, necessary for migration out of Africa. However, some research showed that: 1) Zanzibar was occupied before island formation during Late Pleistocene (Prendergast et al., 2016), 2) Malagasy people share recent common ancestors with Bantu and Austronesian populations, but it is unclear whether Austronesians were the first to settle Madagascar before the Bantus (Pierron et al., 2017) or whether such evidence is weak (Crowther et al., 2016).
Domestication, a hallmark of modern behavior, requires planning and complex cognition. Sub-Saharan Africa shows no evidence of early domestication; cattle-keeping tribes like the Zulu and Maasai likely learned it from Arabs. In contrast, wolves were domesticated in Northeast Asia 100,000-130,000 years ago, and cats in the Middle East 130,000 years ago. Various genetic analyses indicate that cattle domestication is likely (Gifford-Gonzalez, 2000), along with the evidence for African pastoralism (Stock & Gifford-Gonzalez, 2013) notably with migration bringing pastoralism to Eastern and Southern Africa (Skoglund et al., 2017), yet the zooarchaeological evidence seems controversial (Gifford-Gonzalez, 2000), and there is a scarcity of cattle remains (Stock & Gifford-Gonzalez, 2013). Based on Baker’s recount (1974, pp. 357-360), it seems doubtful that Africans ever domesticated these animals.
Systematic hunting, involving foresight and tools, predates the Out of Africa theory. Evidence includes elephant hunts in Britain 400,000 years ago and 400,000-year-old wooden spears in Germany. Fire control, crucial for cooking and defense, appeared in Africa only 60,000 years ago, while Eurasians mastered it 790,000 years ago in Israel. Other research indicate however the presence of stone points at Kathu Pan (500 kya) that functioned as spear tips (Wilkins et al., 2012), some evidence of fire associated with Early Stone Age archaeological material in the form of heated basalt (potlids flakes), heated chert, heated bone, and heated rubified sediment in Kenya (Hlubik et al., 2017), some evidence that fire is way more ancient in Africa than in Israel (Gowlett, 2016).
Chapter 19 – MtDNA
The “African Eve” theory posits that all modern humans descend from a single ancestral mother in Africa around 150,000 years ago, based on mitochondrial DNA (mtDNA) analysis. Afrocentrists argue that Africans have the most diverse mtDNA, suggesting they are the oldest population. Several literature reviews provide strong support for the OoA theory (Nielsen et al., 2017) but without excluding the possibility of the Eurasian origin for modern humans (Bergström et al., 2021). The OoA still faces significant challenges:
Phylogenetic trees and parsimony: Scientists used mtDNA to create phylogenetic trees, assuming the simplest tree with the fewest mutations would show human evolution. The initial tree placed Africans at the base, supporting the Out of Africa (OoA) theory. However, mathematician Henry Gee and others found over a billion equally parsimonious trees, invalidating the claim that the OoA tree was the simplest. Mark Stoneking, a key researcher, acknowledged the theory’s flaws. There are many unresolved issues (Bednarik, 2013), but the greatest weakness of the African Eve model is its reliance on the unrealistic Molecular Clock theory and the Infinite Site Model of mutation (Tomkins & Bergman, 2015; Xia et al., 2021; Zhang & Huang, 2019) owing to the existence of common, recurrent mutations which invalidate the ISM, and even relaxed clock models empirically fail (Tomkins & Bergman, 2015) and can not explain the genetic equidistance phenomenon (GEP) which contradicts both the strict and relaxed clocks (Huang, 2025b).
Mutation rate and coalescence date: Calculating when “Eve” lived relies on assumptions about mutation rates and the accuracy of SNP counts. Errors can arise from paternal mtDNA entering the egg (Hagelberg, 2003; biparental mtDNA inheritance is real but extremely rare; Luo et al., 2018), reverse mutations, or environmental factors like cosmic rays and industrial mutagens, or purifying selection (Henn et al., 2009), which distort mutation rates (Bednarik, 2013). These uncertainties potentially make the coalescence date unreliable. Despite this, two studies estimated a consistent coalescence at 143 kya (Rieux et al., 2014) and 157 kya (Fu et al., 2013).
Founder effect and genetic diversity: Afrocentrists claim Africans’ greater genetic diversity proves they are the oldest population. Fuerle argued that this diversity could result from interbreeding with non-African hominoids who migrated into Africa, rather than being the ancestral population. It would seem that migration after the initial founder expansion was sufficiently limited and admixture with archaics was minimal (Henn et al., 2012b) although the greater genetic diversity may also be partially a result of ancient admixture with yet-to-be identified archaic population(s) (Gomez et al., 2014; Hammer et al., 2011; Wall et al., 2016). Another proposition is that the Founder effect confirms OoA. The reasoning is that, since DNA (both nuclear and mtDNA) gradually mutates, a population will gradually accumulate more variation as it ages. According to Fuerle, “the fact that one population is older than another population does not imply that they are related as progenitors and descendants; a young insect did not descend from an old reptile”. Moreover, if Africans had historically faster generation time, this would explain their higher mtDNA diversity and undercut the validity of OoA (Jeanson, 2016). But perhaps the most devastating criticism comes from the validation of the MGD theory, which undercuts the molecular clock theory assumed by the OoA model. MGD predicts that mutations are a function of biological complexity, whereas the molecular clock predicts that mutations accumulate linearly over time; yet there is ample evidence confirming MGD and invalidating the molecular clock (Huang, 2025b). MGD posits that different groups may show different Maximum Genetic Diversity (MGD) and human groups today are at saturation level of genetic diversity after a very long evolutionary time, especially for the fast evolving DNAs (Huang, 2025b; Xia et al., 2021; Zhang & Huang, 2019), and this implies that diversity differences cannot be explained by time. Africans do have the highest genetic diversity but only in fast changing variants and deleterious variants (Zhang & Huang, 2019).
Chapter 20 – Population Differences in MtDNA
Genes have multiple alleles, which can code for the same trait (e.g., eye color). Allele frequencies vary by population; for example, blue eyes are common in Europeans but absent in Africans and Asians. Groups of inherited alleles form haplotypes, which combine into haplogroups and then macrohaplogroups. Two major mtDNA macrohaplogroups, M and N, include most Eurasians but very few Africans. M dominantly encompasses South and Southeast Asians and has an Asian origin (Chandrasekar et al., 2009; Rajkumar et al., 2005), while N includes West Eurasian (Olivieri et al., 2006).
Population crashes caused by the Toba eruption and ice ages led to bottlenecks, reducing genetic diversity in Eurasia. Survivors in the M and N macrohaplogroups repopulated Asia and Europe around 65,000 years ago. This bottleneck increased average IQ, as smarter individuals were more likely to survive. Africans, unaffected by these events, retained greater genetic diversity due to more stable, humid conditions and larger populations. While population bottleneck (associated with the founding of non-African populations) during the migration of modern humans out of Africa may have influenced the differential pattern of selection observed in African and non-African populations (Campbell & Tishkoff, 2008), and that Toba coincided with the Out of Africa exit (Soares et al., 2012), there is no evidence that Toba caused a bottleneck (Lane et al., 2013).
A phylogenetic tree shows Europeans in the N macrohaplogroup, while South Indians, Pacific Islanders, and Australians have lineages in both M and N, suggesting multiple migrations. Africans, absent from M and N, are genetically closest to chimpanzees, particularly Nigerians (Deka et al., 1995, Table 6). Fuerle argued that if the Out of Africa (OoA) theory is correct, Africans evolved into modern humans but stopped evolving, while Eurasians continued to diverge; this would explain why Africans are both the most primitive and the first to evolve directly from apes to modern humans.
The M and N mtDNA macrohaplogroups, which include most Eurasians, emerged 65,000 years ago, long after modern humans appeared 160,000 years ago. If the Out of Africa (OoA) theory were correct, M and N should have originated in Africa and spread outward. However, these haplogroups are nearly absent in Africans, except in Northeast Africa (e.g., Ethiopia), due to Eurasian migrations into the region. The haplogroups M1 and U6 originate in Asia, and they are mainly found among Northern and Eastern Africans, suggesting a back migration route from Eurasia into North Africa due to the ice age ~30,000 years ago (Gonzalez et al., 2007; Olivieri et al., 2006). However, this model of a migration into North Africa has been contested because: 1) one has yet to find some novel ‘earlier’ Southwest Asian-specific clades, distinct from the known haplogroups, in which M1 and U6 (African-specific subclades of haplogroups) lineages are nested, 2) archaeological evidence doesn’t support a migration from the Levant (Near East) into North Africa as early as 40+ kya, 3) the dates for M1 and U6 subclades are too young (~20 kya) (Pennarun et al., 2012). Moreover, the clades M and N are both derivative branches of the African haplogroup L3 yet it is unclear whether L3 has Eurasian origin (Cabrera et al., 2018) or African origin (Soares et al., 2012). Finally, deep roots of M in India have been established (Chandrasekar et al., 2009), yet if M originates in India it is unclear why in this case the basal L3 lineages (which gave rise to M and N) are absent in India.
According to Fuerle, “the mtDNA of today’s Eurasians has less variation than African mtDNA not because Eurasians are younger than Africans, but because female Eurasians who had mtDNA that was not in the M and N macrohaplogroups did not survive the ice ages.” This could explain why basal L3 existed in early Eurasians and later vanished (Cabrera et al., 2018) but not why the oldest European samples (35 kya, i.e., pre-LGM, Last Glacial Maximum) displayed only M or N derivatives while the later Europeans lost M but not N (Posth et al., 2016).
The mtDNA of Mungo Man (40,000-62,000 years old) from Australia, LM3, is the oldest known Hss mtDNA and does not match modern humans or Neanderthals. This suggests an archaic Eurasian origin, challenging OoA (Adcock et al., 2001). LM3 is linked to a nuclear DNA insertion found in half of Eurasians today, indicating it spread from a single individual in an ancient Eurasian population. But this result relied on Adcock et al.’s study which was riddled with contamination issues and PCR artifacts (Heupink et al., 2016).
Native Americans share haplogroups A, B, C, and D with Siberians, indicating migration via the Bering Strait. Haplogroup X, found in Europeans and some Native Americans, suggests early European migration to the Americas, possibly via a northern route. This evidence points to Europeans being among the first Americans. However, East Asians (from northern coastal China) might in fact be the first settlers of the Americas, alongside Siberians (Li et al., 2023).
Chapter 21 – Nuclear DNA
Humans possess around 25,000 genes, with an average of 14 alleles per gene, resulting in approximately 350,000 nuclear DNA alleles. Most alleles are shared across populations, but their frequency varies. Some alleles are unique to specific populations, such as Europeans, Africans, or Asians. It is unlikely that alleles exclusive to Europeans or Asians today originated in Africans, as this would require an allele initially beneficial in Africa to become harmful there while remaining beneficial in Eurasia, despite a stable environment. Alleles specific to a population likely originated within that group. For example, red and blond hair alleles are unique to Europeans, who have more MC1R gene variants than Africans. Africans possess only synonymous MC1R alleles, encoding eumelanin (black pigment), while Eurasians have additional alleles for pheomelanin (red-yellow pigment), which is absent in Africans due to its disadvantage in Africa. Yet it is possible for some new alleles to spread rapidly due to selection, such as the human EDAR V370A allele, which is a new mutation that arose in East Asia, ~30 kya, i.e., after OoA migration (Kamberov et al., 2013), or perhaps ~20 kya (Hlusko et al., 2018), that is, right after the Last Glacial Maximum (Mao et al., 2021). On the other hand, Fuerle argues that, for OoA to be correct, not only must all of the African-specific alleles disappear from all the Eurasian populations in 65,000 years, but a whole new collection of Eurasian-specific alleles must arise within that time, which is not possible for the entire collection of European and Asian specific alleles.
The spread of Eurasian alleles would have required far longer than the 65,000 years proposed by the Out of Africa (OoA) theory. Harding et al. (2000) estimated that at least 100,000 years were needed for one allele to reach its current frequency. The last common ancestor (LCA) of Africans and non-Africans for the MC1R gene dates back 1 million years, far exceeding OoA’s timeline. Similarly, PDHA1 gene variants indicate population subdivision around 200,000 years ago, with a gene tree dating back 1.86 million years (Harris & Hey, 1999). The different versions of PDHA1 fell into a tree that branched 1.8 mya, one branch of which branched again 200,000 ya. In light of this, Fuerle concludes that if all humans were a single group in Africa 65,000 ya, it would not be possible for there to be humans alive today who have versions of a haplotype that branched twice before that date, yet such haplotypes exist. Fuerle focused on an older version of OoA dismissing admixture, yet evidence suggests the possibility of ancient splits in African hominins and later archaic admixture, which could explain why “hominins with a combination of archaic and modern features persist in the fossil record across sub-Saharan Africa and the Middle East until after ≈35 kya” (Hammer et al., 2011).
Mitochondrial DNA (mtDNA) and Y-chromosome DNA track maternal and paternal lineages, respectively, showing differing migration patterns. Men often migrated without their women, then mated with native women, leading to descendants with native mtDNA and explorers’ Y-DNA (see, e.g., Poznik et al., 2016, Figure 4). Fuerle suggests that Y-chromosome variations indicate limited African migration outside the continent, with unique African variants suggesting no significant OoA migrations. However, some research shows that major Eurasian Y-haplogroups (e.g., CF, DE) descend from ancestral African lineages (e.g., CT), with a TMRCA of ~76 kya for non-African Y-DNA, which is consistent with OoA timelines yet inconsistent with a single OoA migration because the haplogroup E (now predominant in Africa) likely originated outside Africa and then back-migrated (Poznik et al., 2016, Figure 2). It was later confirmed that the OoA model positing a single migration is untenable (Hershkovitz et al., 2018; Posth et al., 2017; Ragsdale et al., 2023). More importantly, the phylogenetic tree of the Out of Africa model is not supported by the data, as the stem haplotypes unique to OoA failed to exhibit the anticipated sharing patterns, whereas the opposite was true for the Out of East Asia model (Huang, 2025a).
Chapter 22 – Replacement
The replacement hypothesis, central to the Out of Africa (OoA) theory, claims that more adapted populations replace less adapted ones. The fact that populations become more adapted to their environment over time makes replacement unlikely. Not only does it imply that tropically-adapted Africans outcompeted the cold-adapted Eurasians in their own environments, but that they replaced them without interbreeding, which is implausible given the hypersexuality of the Africans. The improbable OoA scenario requires the Asian erectus, with his shoveled incisors, to be forced into extinction by modern Africans, who lacked shoveled incisors, but managed to evolve them once they arrived in Asia.
If the first modern men (Hss) arose in Africa, they should have replaced archaic humans (Hs) in Africa first, which requires that they retained all the traits that were advantageous (as well as neutral) in Africa. Yet, due to living in the same environment, the Hss would be very similar to his predecessor. Fuerle argues that if a significant number of today’s Africans do not have modern hard and soft tissue, behavioral, and other traits (especially neutral traits), then neither did the first modern Africans (Hss) who supposedly replaced everyone in Eurasia. Moreover, the Toba eruption and the ice age around 65,000 years ago made Eurasia inhospitable, further undermining the idea of an African migration. Neanderthals, adapted to cold climates with advanced tools and weapons, coexisted with humans until 40,000 years ago, making their complete replacement by Africans improbable. Genetic evidence also contradicts the original OoA thesis. As much as 80% of nuclear loci have assimilated genetic material from non-African archaic humans, indicating interbreeding rather than replacement (Eswaran et al., 2005). The DNA evidence showed extensive introgression between modern and archaic lineages, even after the mitochondrial ancestral lineage had left the African continent, and fossil evidence also support the multiregional (i.e., hybridization) hypothesis (Arnold & Meyer, 2006, p. 272). In fact, the initial Neanderthal admixture could have been as high as ~10%, but negative selection reduced it to present-day levels, i.e., ~2.5% (Harris & Nielsen, 2016). On the other hand, a more flexible version of OoA admits that there is some degree of archaic admixture (Relethford, 2008), with (indirect) evidence of gene flow across African populations (Hublin et al., 2017; Scerri et al., 2018; Schlebusch et al., 2017), thus extending the original OoA to what is called the “African multiregionalism” (Scerri et al., 2018). Even if we accept this revised model, OoA’s clean replacement narrative is complicated by the finding that the archaic Denisovans were widespread in East Asia and that Middle Pleistocene crania simultaneously possess both ‘modern’ and ‘primitive’ traits, suggesting gradual assimilation rather than replacement (Liu & Wu, 2022; see also, Gao et al., 2017; Wu et al., 2023).
Alleles for neutral traits persist for millions of years, yet OoA implies Africans lost all African-specific traits in just 65,000 years. Eurasian-specific traits, like light skin and hair, are recessive and would not spread widely if Africans replaced Eurasians, even though these traits may be acquired through interbreeding with Neanderthals (Sankararaman et al., 2014; Vernot & Akey, 2014) and even though some simulations suggested that the onset of selective sweeps (rapid increase in frequency) for Eurasian-specific alleles associated with skin color occurred after the OoA migration (Beleza et al., 2013). Africans losing African alleles would not automatically gain Eurasian traits; for example, losing black skin would result in albinism, not white skin. During the Out of Africa expansion, people losing their African-specific traits would have been at a severe disadvantage until advantageous mutations occur. Even if we assume that the Africans passed through tropical Asia, they would not evolve Eurasian traits, because the environment was similar to Africa. Following this, the Negritos should resemble Africans, and they do indeed, yet contra OoA, the Negritos are genetically closer to Eurasians than Africans (Jinam et al., 2017).
Cro-Magnons, with a skull resembling modern Caucasians, lived in Europe 32,000 years ago, leaving Africans only 33,000 years to evolve similar traits—an implausibly short timeframe. Evolution typically moves from generalized to specialized traits, therefore Eurasians would not have evolved from Africans. Africans have specialized traits such as African hair that keeps the brain cool, and is thus specialized by its shortness, cross-sectional flatness, and the absence of a central duct, which makes it wooly. Yet Africans also possess traits maladaptive in Africa, like external nasal bones, suggesting migrations of early man into Africa; indeed, in the tropics, where the air is warm, there is no need for nose bones to support a large nose to warm the air, and apes do not have them. The large size of Africans is also another hint at the migration of northerners into Africa because, according to Bergmann’s Rule, Africans should be small; all pygmies and Negritos and Hobbits are tropical and small. But Fuerle fails to realize that Bergmann’s rule is not universal, and this rule does not hold in the southern hemisphere (Foster & Collard, 2013). In fact, African populations may have higher genetic endowment for body height, as evidenced by their higher than average polygenic scores (Piffer & Kirkegaard, 2024). The short stature of pygmies is likely due to selection pressures for adapting to local environments and limited food resources (Fan et al., 2016).
SECTION 4 – The Out-of-Eurasia Theory
OoE theory suggests that human ancestors likely originated in Asia, with major human lineages (Africans, Neanderthals, Caucasians, Asians) splitting over 2 million years ago. OoA assumes Africans evolved independently, while OoE argues African evolution was influenced by repeated Eurasian migrations and interbreeding. Intelligence is the driving force of evolution, with key stages being bipedalism (which frees hands for tool use, creating a feedback loop of larger brains which cause better tools, which cause more resources, which cause larger brains) and migration to colder climates (which intensifies selection for intelligence due to seasonal challenges such as winter survival, hunting, planning, cooperation and the need for technology such as fire, clothing, shelters). In light of this, Fuerle doubts that the modern Africans who migrated out 65,000 years ago (and who likely have an IQ even lower than today’s Africans) have increased their IQ by 2 SD. Counterintuitively, OoA requires high intelligence to have a greater optimum in Africa. Fuerle’s take on OoE aligns with Begun’s (2016).
As of late, there is some evidence supporting OoE. Recent reconstructions of Mediterranean C4 grasslands (~7.2 Ma) show that savanna habitats (assumed to be critical for early hominin evolution) existed in Eurasia, and that the Graecopithecus (~7.2 Ma) from Greece may be the oldest known hominin, predating the next youngest candidate (African) hominin Sahelanthropus (~6-7 Ma), which implies the Pan-Homo split occurred in Europe, not Africa (Böhme et al., 2017; see also, Fuss et al., 2017). Late Miocene apes from Anatolia (e.g., Anadoluvius, 8.7 Ma) suggest hominines may have first diversified in Eurasia, challenging the assumption of an exclusively African origin for the human-ape lineage (Sevim-Erol et al., 2023). Multiple lines of evidence indicate that the last common ancestor (LCA) of humans and chimpanzees lived in Eurasia, migrated to Africa during the Messinian Salinity Crisis (~5.9-5.3 Ma), while proto-Homo became isolated on the Red Sea coast after the Zanclean flood (5.3 Ma), who evolved aquatic adaptations before dispersing globally after 2.6 Ma due to the drop in sea-levels in the early Pleistocene (Mansfield & Vaneechoutte, 2024). This scenario is even more plausible than the savanna hypothesis because evolutionary changes that distinguish humans from other primates is the result of a semi-aquatic or coastal lifestyle, causing loss of body hair, increased subcutaneous fat, encephalization, eccrine gland proliferation, multi-pyramidal lobulated kidneys, loss of olfactory ability, voluntary breath control, an improved diving response, larger, more streamlined bodies, concealed ovulation, etc.
Chapter 23 – The Bipedal Apes
The transition to bipedalism likely began in Asia. This is because, before there were any bipedal apes, tree-dwelling mammals were being selected for increased intelligence in both Africa and Asia. Recent evidence indeed suggests that the arboreal environment was an essential component of the early hominin evolution, refuting by the same token the savanna hypothesis (Antón et al., 2014; Drummond-Clarke et al., 2022; Mansfield & Vaneechoutte, 2024). Early primates like Teilhardina (55.5 million years ago) and Bahinia (40 million years ago) originated in Asia, with tarsiers—potential ancestors of bipedal hominoids—also found in Asia, Europe, and North America, but not Africa. The absence of horizontal branches in Asian forests may have favored upright postures, leading to bipedalism. Fuerle showed that fossils like Dryopithecus (20-9 million years ago), found in Europe, Asia, and Africa, display traits closer to Asian apes and humans than African apes, suggesting a Eurasian origin for great apes. Nonetheless, Begun (2016, pp. 154-155) enumerated some features of Dryopithecus that are similar to those of African apes.
The first partially bipedal ape, Oreopithecus (11.2-3.4 million years ago), lived in Europe and West Asia, with fossils also found in Africa, implying migration from the north. Oreopithecus had human-like features (Moyà-Solà et al., 1999, 2005) and likely waded in water (Niemitz, 2010), facilitating the transition to terrestrial bipedalism (Kuliukas, 2002, 2012, 2014). This aligns with the aquatic ape hypothesis (Corballis, 1993, pp. 56-58; Mansfield & Vaneechoutte, 2024; Vaneechoutte et al., 2012a, 2012b), as water reduced leg weight and improved balance. Australopithecus, a fully bipedal descendant of Oreopithecus, emerged in Africa but may have originated in Southwest Asia, given the sudden appearance of hominins in Africa and the lack of pre-Pleistocene ancestors there (Coon, 1962, p. 217).
African apes (chimpanzees and gorillas) knuckle-walk, while Asian apes (e.g., orangutans) palm-walk. Humans, with generalized traits, likely evolved from a Eurasian ape like Oreopithecus, which waded in water, rather than specialized African knuckle-walkers. Wrist bone analyses show early Australopithecus closer to knuckle-walkers, but later specimens were equidistant between African apes and orangutans, with humans overlapping more with orangutans. This suggests human ancestors may have lost knuckle-walking adaptations, which is counterintuitive given the rule that evolution goes from generalization to specialization, and there is no evidence that any known hominin was a knuckle-walker (Begun, 2016, p. 219). Another possibility is that a quadrupedal African ape interbred with a bipedal Eurasian ape that had migrated into Africa.
Most paleoanthropologists argue humans descended from a chimpanzee-like ancestor due to closer genetic similarity, but evidence suggests humans may instead share a more recent common ancestor with orangutans. While humans and chimpanzees have a smaller genetic distance, this may result from interbreeding between the Homo lineage and chimpanzees (Patterson et al., 2006) especially after the Homo lineage migrated to Africa. On the other hand, humans and orangutans share more morphological and behavioral traits, such as thick tooth enamel, reproductive behaviors, and brain asymmetry. Corroborated documentation of traits reveals that humans and African apes share 8 traits while humans and orangutans share 28 traits (Grehan, 2006, Tables 1-2). On top of that, the mechanics of orangutan bipedalism are more similar to those of modern humans than are those of panins and gorillines (Thorpe et al., 2007).
Orangutans exhibit many human-like traits, including bipedal adaptations (e.g., a higher sacral index and human-like femur), neoteny, flat faces, and advanced cognitive abilities like tool use and cultural behaviors (van Schaik et al., 2003, 2009). They also share reproductive traits with humans, such as concealed ovulation, private mating (by front-to-front instead of front-to-back like chimpanzees and bonobos do), and pair bonding, unlike chimpanzees. Even more interesting: orangutans and humans share unique features like beards, closed-mouth smiles, and right-handedness.
The bonobo, a chimpanzee relative, also shows human-like traits (de Waal & Lanting, 1997), such as longer legs, frequent bipedalism, and neoteny, possibly due to interbreeding with a bipedal ape ancestor. Despite being knuckle-walkers, bonobos exhibit more human-like behaviors and physical traits than common chimpanzees.
Chapter 24 – The Origin of the Eurasians
The evolution of human lineages likely began with Australopithecus, a generalized ancestor that adapted to different climates, rather than the earlier Oreopithecus. The Australopithecus diversified into tropical and cold-adapted lineages. The cold-adapted populations evolved in two ways: Neanderthals in Europe and West Asia developed robust, hairy bodies, while East Asians became neotenous, with traits like subcutaneous fat (Wells, 2016, p. 185) and reduced body hair for cold protection. These generalized Australopithecus persisted in intermediate regions, advancing socially and technologically rather than anatomically.
Most of the early evolution of Caucasians is believed to have occurred in India, then in South West Asia, and finally in Eastern Europe. Europeans are considered the most generalized of the major races, suggesting their lineage predates specialized adaptations. Despite fewer genetic variations due to population bottlenecks caused by events like the Toba eruption (although contested) and ice ages, Europeans likely descended from generalized Australopithecus. In contrast, East Asians accumulated more genetic diversity, because the Europeans were decimated much more by Toba and the ice ages. Fuerle speculated that “All three of the northern populations (Neanderthals, West Asians, and East Asians) were becoming more intelligent as they moved farther north, and the generalized West Asians were becoming more innovative because they were less selected for anatomical cold adaptations and therefore had to rely more upon technology to survive in the cold.”
Georgicus is a good example of what early Homo, adapted to the cold, might have looked like. Georgicus is, in some ways, very similar to early types of Homo (habilis and ergaster, found in Africa) and later erectus, so much so that some scientists classify georgicus as an ergaster, while others place it among the erectus. Georgia had an alpine climate in the mountains (north and southwest borders) and a subtropical climate on the Black Sea. So early men (e.g., georgicus) could have foraged and hunted in the mountains during the summer, then retreated to warmer plains in the winter, gradually evolving into a population more anatomically adapted to the cold.
Like other populations moving north, the Neanderthal lineage (Georgicus, Antecessor, Heidi, and the Neanderthal man) became larger, stronger than their southern neighbors, and it again expanded into warmer climates (Africa included, to a lesser degree). Tropical natives were better adapted to the tropics than northern populations, which were absorbed and went extinct. After the human revolution (50,000 years ago), West Asians were able to expand, move north, and in the process pushed Neanderthals out of Georgia and Eastern Europe.
Early humans likely lost most body hair around 240,000 years ago due to a genetic change (deactivation of the KRT41P gene) (Klein & Takahata, 2002, p. 200), which spread as less hair became a sign of health and reduced disease risks. This adaptation allowed better heat dissipation in tropical climates and increased vitamin D production in colder regions (Jablonski, 2021). Early humans, using animal skins for clothing, faced fewer parasites and diseases, favoring less hairy individuals.
The generalized Homo sapiens (Hs) migrated through both northern and southern routes around the Himalayas, settling in erectus-free areas of Asia first. Advanced populations (Hss) later pushed generalized Hs further south, where some interbred with erectus in regions like New Guinea and Australia. In West Asia, the generalized Hs lacked cold-adapted anatomy but used fire and tools to survive seasonal changes. This adaptability marked the transition from archaic to modern humans (Hss), exemplified by the Cro-Magnons in Europe.
During the second ice age (30,000-12,000 years ago), large herbivores declined, impacting Neanderthals and Aurignacians. Aurignacians migrated to Europe around 35,000 years ago, followed by Gravettians from the Middle East 25,000 years ago, who had better cold-adapted cultures. The Cro-Magnon splitted from the Neanderthal over 2 mya but there was still some interbreeding. The Cro-Magnons absorbed and displaced the Neanderthals (46,000-24,500 years ago) while they were absorbed and displaced by the Mongoloids. Some northern Mongoloid/Cro-Magnon hybrids migrated to the Americas, becoming the Native Americans. This interbreeding complicates genetic timelines, making common ancestors appear more recent.
In line with OoE, genetic distances show East Asians evolved further from the original Hss than Europeans. Specifically, Africans and Asians specialized in opposite directions while Europeans remained generalized. Thus, Asians are genetically farther from Africans than Europeans. This contradicts OoA, which would predict closer African-Asian ties because Africans would migrate to Asia first. Nevertheless, it is worth noting that bottlenecks during the northward expansion of East Asians post-LGM (Sikora et al., 2019) would have drastically increased their genetic distance through drift and that their higher Neanderthal admixture (Sankararaman et al., 2014, 2016) would also act to increase their distance relative to Africans.
The Ainu, indigenous to Japan, are remnants of interbreeding between East Asians and the Jomon, a maritime people (Jeong et al., 2016; see also, Cavalli-Sforza et al., 1994, p. 203). They retain primitive Hs features, such as prominent brow ridges and large teeth, but have paler skin and some blue eyes. Their hairiness and language similarities to Basque (Nyland, 2001) suggest isolation from East Asians and a connection to ancient West Asian Hs.
Chapter 25 – The Neanderthals
Between 350,000 and 24,500 years ago, Neanderthals occupied Europe, and created tools, ornaments, and even musical instruments. They cared for their wounded and buried their dead, but their numbers declined as Cro-Magnons arrived around 46,000 years ago. While the Neanderthals were ambush hunters, relying on strength and stealth, the Cro-Magnons used lighter spears, followed migratory herds, and had dogs, giving them a hunting advantage. And the Cro-Magnons also had better communication, social networks, and artistic expression, which contributed to their survival as Neanderthals, specialized for hunting large mammals, went extinct due to dwindling prey.
Given the principle that males of the more advanced and expanding population mate with the females of the less advanced population, interbreeding between Cro-Magnons and Neanderthals likely occurred, with male Cro-Magnons mating with Neanderthal women. This introduced Neanderthal DNA into the Cro-Magnon gene pool, leading to hybrid offspring with a mix of traits (typically the alleles which retain their selective advantage in the face of an invasion by another race; see Coon, 1962, p. 34). Then these hybrids become the more adapted population and now the hybrid males can take either the more primitive-looking Neanderthal females or the more feminine-looking Cro-Magnon females; likely the latter group. This makes theoretical sense. It is therefore surprising that the opposite was true, with a bias toward Neanderthal men mating with modern women and the reduced fitness of Neanderthal women x modern men hybrids (Juric et al., 2016).
Over time, these hybrids, combining Cro-Magnon cognitive abilities and Neanderthal physical strength, became dominant, eventually forming the Caucasian population. Despite the failure to find Neanderthal mtDNA in Europeans, Fuerle proposed some (speculative) evidence. Northern Neanderthals differ genetically from southern Neanderthals (Rosas et al., 2006), and this hints at the possibility that Cro-Magnons in the north interbred with northern Neanderthals while other genetically-similar Cro-Magnons in the south interbred with genetically-different southern Neanderthals. Moreover, there is some genetic evidence for a 900,000 base pair inversion (i.e., the DNA string is backwards) in Chromosome 17 that is at least 3 myrs old (Stefansson et al., 2005). The mutation confers a reproductive advantage and is found in about 20% of the Caucasians, but is almost absent in Asians and is rare in Africans. Fuerle’s interpretation is that the inversion arose before the LCA in an Australopithecus that was in the Neanderthal lineage and the Cro-Magnons who migrated into Europe interbred with Neanderthals who had the mutation, giving it to their hybrid children, the Caucasians. And later, some Caucasians gave it to a few Africans and Asians.
Neanderthal contributions to Caucasians (through interbreeding) include physical traits like midfacial prognathism (protruding nose and jaw) and perhaps even genetic traits like the DRD4-7R allele (Ding et al., 2002), associated with novelty-seeking behavior. These traits, along with others like blue eyes and blonde hair, likely spread through genetic hitchhiking, where non-advantageous alleles were passed on alongside advantageous ones. This hypothesis is at best partially validated, due to odd patterns in allele frequencies between Europeans and East Asians (Reilly et al., 2022). The Neanderthal contribution is likely neutral, meaning that Neanderthals themselves had diverse pigmentation traits (Dannemann & Kelso, 2017). To complicate matters, some studies indicate that light pigmentation alleles (at TYRP1 gene, which is linked to iris pigmentation and hair color within Europe) swept to high frequency in Europe after OoA (Beleza et al., 2013) and that a common founder mutation in the OCA2 gene (the major contributor to the eye color variation) was responsible for the blue eye color (Eiberg et al., 2008). More importantly, sexual selection for european hair, eye and skin color may be a more plausible explanation (Frost, 2014a). On the other hand, some Neanderthal alleles were beneficial and provided functional genetic diversity (including skin phenotypes) that helped modern humans adapt to new environments (Iasi et al., 2024; Racimo et al., 2015; Sankararaman et al., 2014; Vernot & Akey, 2014). Moreover, fossil evidence seems to support Neanderthal-Eurasian interbreeding, such as the presence of a “horizontal-oval” mandibular foramen, a trait which has no clear functional significance and yet is unlikely to have arisen by chance.
The OoA theory struggles to explain the unique traits of Caucasians (e.g., why they are less neotenic than Asians even though they both lived in a similar environment), which are better explained by Neanderthal admixture. It was indeed observed that “Neanderthal faces are essentially Caucasoid” (Coon, 1962, p. 534). Similarly, the presence of blonde hair and blue eyes in some Australian Aboriginals suggests ancient interactions with Neanderthal-like ancestors or early Caucasians. Yet at the same time, the Neanderthal admixture is noticeably higher among East Asians (Vernot & Akey, 2015).
Chapter 26 – The Origin of Africans
Unlike Eurasians, who evolved far from their primate ancestors, the Africans remained in environments similar to those of early primates, leading to fewer advantageous mutations. Still, there were multiple waves of migration within Africa (Tishkoff et al., 2009), causing the migrating populations to face novel environments and selective pressures, resulting in local adaptation (Fan et al., 2019; Scheinfeldt et al., 2019). Fuerle believes that Africans are largely shaped by migrations and interbreeding with Eurasian hominins, who brought advanced traits and culture. Without these migrations, Africa would lack members of the Homo genus today. Although a recent study found that Eurasian ancestral populations contributed as much as a third of the genetic material in many modern African populations due to back-migration after OoA, the applied method had little power to detect migration predating 70 kya (Cole et al., 2020).
Eurasian hominins, including bipedal apes, Australopithecus, Homo erectus, and later Homo sapiens, migrated to Africa over millions of years. These migrants interbred with indigenous populations, creating hybrids with a mix of Eurasian and African traits. Over time, more advanced migrants pushed earlier populations into less desirable regions, where they persisted before eventually dying out. The African genome has been shaped by outside migrants. Even if the Eurasian migrants were absorbed, they brought with them a set of alleles, meaning that the negative effects of a few alleles in the set that were maladaptive in Africa may have been swamped by the positive effects of the remaining adaptive alleles in the package.
The Sahara Desert acted as a barrier, limiting migrations except during ice ages when it was more habitable, thanks to humid periods (Drake et al., 2011). The white population in North Africa could thus move south, entering the Horn of Africa into Ethiopia.
Some African populations are less evolved, due to their geography. Congoids, the most simian group, likely descended from the earliest hominoids in Africa due to their area being inaccessible to Eurasian migrants. Their tropical traits, such as dark skin and curly hair, may have originated from bipedal monkeys or Australopithecus. The pygmies, who live in forested areas of the Congo, are characterized by their short stature, reminiscent of the short stature of Australopithecus. The Nigerians are the African tribe that is genetically closest to the chimpanzee (Deka et al., 1995, Table 6). Nigeria is on the West Coast of Africa, making it difficult to reach from the Middle East. This could explain why Nigerians received fewer infusions of Homo genes from Eurasians.
Other African populations display some hybrid features. The San (Bushmen) and Hottentots (Khoi) of southern Africa have been shaped by ancient migrations and interbreeding events. One of their unique traits, e.g., steatopygia (enlarged buttocks), suggest descent from a tropical-adapted Australopithecus population in India, which migrated to Africa during ice ages. The Hottentots, closely related to the Bushmen, carry the oldest human Y-chromosome haplogroup A (Knight et al., 2003) and exhibit steatopygia, a trait also found in Andaman Islanders, linking these groups to a common ancestor. The Bushmen are not only monogamous, a trait of the cold north, but they also display both African traits, such as thick lips, curly hair, and Asian neotenic traits, such as light skin, epicanthic folds as well as other infantile features (Baker, 1974, p. 312), which led Fuerle to suspect interbreeding between the steatopygous Andaman Islander lineage and the neotenic East Asian lineage. However Fuerle did not consider the alternative explanation of these traits resulting from either adaptation or sexual selection (for a counterpoint, see, e.g., Lin et al., 2018). And one problem with the ancient admixture statement is that the eurasian admixture among Khoisans was very recent, and was observed after the OoA event (Pickrell et al., 2014; Schlebusch et al., 2017). Another one is that southern African Khoesan populations have the most diverged genetic lineages, likely due to long term isolation, >200 kya (Fan et al., 2023), until very recently (Tishkoff et al., 2009; Schlebusch et al., 2012). Fuerle’s hypothesis is not completely refuted yet, owing to the evidence of multiple introgression events from “ghost” populations with highly diverged genetic lineages (Fan et al., 2023).
East Africans show less simian traits due to interbreeding with more recent Eurasian migrants, including Cro-Magnons pushed south by ice ages. The contribution of West Eurasian ancestry in Eastern Africa across various populations is not negligible although huge variations are observed (Haber et al., 2016; Pagani et al., 2012; Pickrell et al., 2014, Table1; see also, Cavalli-Sforza et al., 1994, p. 174, & Hodgson et al., 2014a, Table 2). Similarly, North Africans have substantial Eurasian ancestry, although with varying sub-Saharan ancestry, ranging from 1% to 55% (Henn et al., 2012a). On the other hand, the West Africans, like Nigerians and Pygmies, are genetically more distant from Eurasians, likely descending from the earliest hominid migrants to Africa.
The Boskop skull, dated at 30,000-10,000 years ago and found in South Africa, exhibits both African and Caucasian traits, and thus suggests some interbreeding between Cro-Magnons and indigenous Africans. Its large cranial capacity (1860 cc) and high forehead contrast with its African-like jaw and cephalic index, but the lack of persistence of such large brains in Africa still implies that optimal intelligence there is lower than in Eurasia. Similarly, the Grimaldi skulls, dated at 30,000 years ago and found on the Mediterranean, show Negroid-Caucasoid hybrid features (Coon, 1962, p. 584), likely because the ice ages drove Cro-Magnons into Africa where they interbred with Africans. When the ice receded, the hybrids advanced north around the Mediterranean. They were later replaced by un-hybridized Cro-Magnons.
Chapter 27 – The Origin of Asian Aborigines
The tropical aboriginal populations, such as Australian Aboriginals, are remnants of tropical-adapted Homo erectus groups pushed south by more advanced Homo sapiens (Hs and Hss) migrating from the north. These groups survived in isolated, less desirable areas like islands, mountains, and dense forests, while interbreeding with northern populations and thus acquiring Hs and Hss traits. The Australian Aboriginals consist of three distinct groups: Pygmies in northern Queensland rainforests, Desert Aboriginals in southern deserts (macrohaplogroup N), and Coastal Aboriginals in northern coastal areas (macrohaplogroup M).
During the first (73,000-55,000 years ago) and second (30,000-12,000 years ago) ice ages, lowered sea levels allowed migrations from Southeast Asia to Australia. The N macrohaplogroup, more advanced and from India and North Asia, arrived first, around 60,000 years ago, while the M macrohaplogroup, more primitive but larger, arrived later during the second ice age, pushing earlier arrivals south. Coastal Aboriginals, more primitive than Desert Aboriginals, retain stronger erectus and tropical-adapted Australopithecus traits, resembling Negritos and Africans with black skin and curly hair. Desert Aboriginals, resembling primitive Caucasians, have lighter skin and wavy or straight hair.
The Australian Pygmies, with their small stature, curly hair, and neoteny, likely descend from a tropical-adapted Asian Australopithecus. The presence of small-stature people in Australia, Africa (Bushmen), and Indonesia (Hobbits) aligns with Bergmann’s rule. Despite lacking fossils, their Australopithecine traits suggest they arrived long before 40,000 years ago.
Coastal Aboriginals exhibit clear erectus characteristics (e.g., a considerable prognathism), as seen in skull comparisons with modern Caucasians. Their genetic distance from Africans indicates a common ancestor, likely a tropical-adapted Australopithecus from over 2 million years ago. The survival of these primitive populations in the South Pacific reflects no severe ice age impacts (Tobler et al., 2017), and seemingly less severe in Asia compared to Europe. This would allow erectus and Australopithecus descendants to persist in isolated regions.
A challenge to Fuerle’s assumptions here is the finding that Aboriginal Australians descend from a single founding population that differentiated ~10-32 kya (Malaspinas et al., 2016; see also, Nielsen et al., 2017), resulting in a single and rapid migration (Tobler et al., 2017), despite evidence of archaic admixture predating the population split between Aboriginal Australians and Papuans (Malaspinas et al., 2016).
SECTION 5 – Policy
Fuerle wonders whether the evolution that has made us what we are today will continue along this path or whether it will reverse, bringing us back to the primitive state we were in a very long time ago. Since Asians have limited ‘foreign’ immigration into their countries, they are not concerned with the problem faced by modern Caucasians. The idea of this book is expressed in its title: Erectus Walks Amongst Us. This means that primitive men live among us (the “us” here refers to White people) in the West. Since miscegenation allows for the transmission of African alleles, the civilized Western world will become more erectus and less sapiens.
Chapter 28 – Homo Africanus
The classification of species is often arbitrary, especially when distinguishing between closely related populations. While interbreeding is a common criterion, many interbreeding populations are classified as separate species (e.g., pintail ducks and mallards, wolves and coyotes). A more useful definition, proposed by Schwartz (1999), suggests that two populations are different species if a genetic improvement in one threatens the survival of the other. This definition is particularly relevant when examining the boundaries between Homo erectus (He), Homo sapiens (Hs), and modern humans (Hss).
Morphology is no longer needed to distinguish between different species, although contra Fuerle, some argued otherwise (de Queiroz, 2007). DNA analyses are arguably a better way to classify species. For example, the genetic distance between sub-Saharan Africans (Bantus) and Eurasians (English) is 0.23%, while the distance between common chimpanzees and bonobos is 0.103%, and the distance between Gorilla gorilla and G. beringei is 0.04% (Guillén et al., 2005, Table 1). According to Fuerle, this raises the question of whether Africans and Eurasians should be classified as separate subspecies, Homo sapiens africanus and Homo sapiens eurasianensis, respectively. It also highlights taxonomists’ inconsistent criteria.
A study reported that the genetic distance between Homo sapiens and Homo neanderthalensis is 0.76, while the genetic distance between Homo sapiens and Homo erectus is 0.190, compared to the genetic distance between sub-Saharan Africans and Eurasians of 0.2% (Curnoe & Thorne, 2003, p. 214). The same study reported that “The mean of 8 other genetic distances between H. sapiens and H. erectus is 0.065–0.068. This overlaps the range of distances for living humans, with the lower estimate identical to the distance between “Bantu” and “Eskimo” (Cavalli-Sforza et al 1994).” Because the African-Eurasian distance is more than twice the Homo sapiens-Neanderthalensis distance, Fuerle argues that these figures support the case for subspecies classification. One issue with these studies though is the reliance on mtDNA, which is affected by mitochondrial introgression, selection and incomplete lineage sorting, leading to inaccurate estimates, especially without confirmation from nuclear data (Dong et al., 2021; Funk & Omland, 2003; Melo-Ferreira et al., 2014; Toews & Brelsford, 2012; Zink & Barrowclough, 2008).
Chapter 29 – Miscegenation
Miscegenation, once prohibited and likened to bestiality, is now promoted in media, though it faces resistance in some cultures, particularly among Asians. Despite increasing rates of interracial births, by 4- to 5-fold from 1968 to 1996 (Getahun et al., 2005), evolution inherently discourages miscegenation, as populations develop traits favoring reproduction within their own group to preserve advantageous adaptations. Miscegenation, likened to entropy in physics, irreversibly mixes unique racial traits, erasing millions of years of evolutionary differentiation. This loss of racial diversity is akin to species extinction, a tragedy for those who value biological and cultural uniqueness. In fact, even animals obey their natural instincts by seeking other animals that are similar to their own kind (Baker, 1974, p. 85).
Egalitarians advocate for diversity in all aspects of society except humans, who must miscegenate and, in doing so, become finally equal. But this does not preserve diversity; this destroys it. Today, the rise in interracial relationships, particularly involving lower-IQ populations, may contribute to a dysgenic effect, by slowly reducing national IQ and GDP over time. Blacks generally favor miscegenation more than whites, as it elevates their social status, while whites and blacks alike tend to reject primitive traits, which blacks often possess. Mixed-race individuals, or “mulattoes,” typically identify as black and align with black interests, reinforcing the preference for miscegenation among blacks. This is partly due to the dominance of black traits in mixed-race individuals, as whites carry more recessive alleles that require two copies to be expressed, such as straight hair or light skin.
Miscegenation, while increasing racial mixing, undermines the genetic uniqueness of populations, particularly Caucasians, leading to a decline in high-IQ individuals who drive economic and societal progress. This should not be taken lightly because the cognitive ability of the cognitive elite does influence economic outcomes over and above that of the average ability (Kirkegaard & Carl, 2022). Fuerle then cites historical examples, such as ancient Egypt, the Arab world, Classical Greece, and Portugal, as an illustration of how miscegenation and the influx of African alleles contributed to the decline of once-great civilizations by depressing their IQs. Yet some of the sources that Fuerle relies on are weak. Among Egyptians, sub-saharan African admixture can go up to 20% (Pagani et al., 2015; Schuenemann et al., 2017; Wohlers et al., 2020), although with great variability (Morez Jacobs et al., 2025), but attributing Egypt’s low IQ primarily to this admixture is farfetched, and PCA showed that Egyptians are closer to the Middle East than to North Africa (Fadhlaoui-Zid et al., 2013). Among Greeks, it was found that there is a genetic continuity between modern Greeks and Mycenaeans, with a non-trivial Anatolian Neolithic-related ancestry, and that Greeks cluster closely with other Europeans (Antonio et al., 2019; Clemente et al., 2021; Lao et al., 2008; Lazaridis et al., 2017; 2022; Novembre et al., 2008). Among Arabs, the Sub-Saharan African admixture is limited (8-9%) in some studies (Al-Zahery et al., 2011; Almarri et al., 2021; Černý et al., 2009) and more substantial (up to 20%) in some (Fernandes et al., 2015; 2019; Hellenthal et al., 2014), yet attributing their low IQ primarily to African admixture is unrealistic. Among Portuguese, mixed race unions were visible throughout Portugal, particularly in the South (Magalhães, 1997), with admixture being indeed higher in the South (Gonzalez et al., 2003; Roca-Rada et al., 2024), reaching 10% or 22% in some cities in the South (Pereira et al., 2010), yet the North of Portugal exhibited a strong genetic continuity during the periods of Islamic and Christian Conquest to the Early Medieval period (Roca-Rada et al., 2024).
In modern contexts, regions with higher levels of miscegenation, such as Brazil and the United States, show stark disparities in wealth, education, and IQ between predominantly white and mixed or black populations. The U.S. Census Bureau predicts that non-Hispanic whites will become a minority by 2042, with Hispanics and other minority groups growing rapidly. This demographic shift, coupled with the dysgenic effects of miscegenation, threatens to reduce the proportion of high-IQ individuals, further accelerating the decline of Western civilization.
As the West faces this decline, the center of global civilization is shifting to East Asia (China, Japan, Singapore, South Korea), where higher average IQs and lower levels of miscegenation support continued advancements in science, technology, and military power. The West, meanwhile, risks becoming mired in economic and intellectual stagnation, unable to reverse the effects of widespread racial mixing and the loss of its genetic and cultural heritage.
Chapter 30 – Hybrid Vigor
Egalitarians claim mixed-race individuals are superior due to “Hybrid Vigor,” where hybrids exhibit greater strength or growth than their parent populations. However, this contradicts their stance that races are genetically equal. Hybrid Vigor occurs when two genetically distinct populations interbreed, creating heterozygous offspring with diverse alleles. Just as inbreeding does not create deleterious recessive alleles (DRAs) but merely exposes them, outbreeding does not exterminate DRAs but merely conceals them (Keller & Waller, 2002; Schwartz, 1999, p. 266).
As isolated populations become more homozygous, advantageous alleles are fixed and harmful alleles eliminated more quickly, making them better adapted to more stable environments. The downside is that loss of genetic variation reduces evolutionary potential in the face of environmental change (Frankham, 2005). Given that a population that has a large number of expressed recessive traits has likely been isolated for a long time, Fuerle argues that “If Eurasians express more recessive alleles than Africans (which seems likely, given that Africans have greater variation), that would lend support to the OoE theory because it would suggest that Eurasians were more isolated than Africans and Africans received their alleles from Eurasians, not the reverse.”
However, miscegenation disrupts this near-optimum adaptation (see Chapter 4 Rule 10) by mixing alleles from different populations, leading to suboptimal genetic combinations (Charlesworth & Willis, 2009; Edmands, 2007). While the first generation of hybrids may benefit from masking DRAs, subsequent generations face higher risks of expressing harmful alleles, reducing their fitness.
True Hybrid Vigor occurs when inbred, purebred populations (homozygous for advantageous alleles) interbreed, producing offspring with enhanced traits like strength and health. This contrasts with mixing heterozygous populations, which results in genetic incompatibility and reduced fitness. Purebred populations are created through inbreeding, causing both desired and undesired traits coded for by recessive alleles to be expressed much more because the probability of two recessive alleles ending up in the same individual is greater. When two purebred populations with different advantageous alleles are crossed, their hybrid offspring exhibit hybrid vigor. However, this vigor dissipates in subsequent generations due to genetic recombination, making sustained hybrid vigor dependent on continuous selective breeding (Simpson, 2003, pp. 601-602).
In nature, hybrids face rigorous selection, with only the fittest surviving. The emergence of Caucasians from Cro-Magnon and Neanderthal hybrids is an example. Modern human societies, however, support the survival and reproduction of less fit hybrids through social and medical services, relaxing natural selection (Lynch, 2016). Miscegenation today disrupts the genetic compatibility of isolated populations, and as a result new deleterious mutations will arise in each generation. Fuerle suspects this can lead to regression to the mean in traits like intelligence, where offspring of mixed-race parents may not inherit the combined advantages of both lineages.
Chapter 31 – Segregation
The freedom of association, though not explicitly stated in the Bill of Rights, was historically implied in the First Amendment until the Civil Rights Act of 1964 effectively abolished it. This act stripped individuals of the right to associate or disassociate with others based on personal preference, despite the Ninth and Tenth Amendments reserving unenumerated rights to the people and states. Today, private racial segregation in housing, schools, workplaces, and public spaces is illegal, yet the innate tendency to associate with genetically similar individuals persists, as seen in friendships, partnerships, and neighborhood choices.
Despite egalitarian pressures, people continue to prioritize racial and cultural identity. Surveys reveal that a significant majority of whites and an even larger proportion of blacks value racial identity and cultural preservation. Blacks exhibit stronger in-group preferences than whites, and even children naturally segregate by race when given the choice. Anti-racists often rationalize living in predominantly white neighborhoods by citing “good schools” and “safe environments,” inadvertently reinforcing racial divides.
This behavior mirrors that of territorial primate species, which form “biological nations” of related individuals and defend their territories against outsiders. Such territorial societies tend to be more egalitarian, with lower dominance hierarchies and less physical conflict, fostering social solidarity and cooperation. Similarly, human tendencies toward racial association and territoriality reflect deep-rooted biological and social instincts, challenging the egalitarian ideal of a race-blind society.
Chapter 32 – Eugenics
Eugenics involves guiding human evolution by selecting desirable traits, as natural evolution lacks a specific goal. While evolution continues naturally, its outcomes may not align with human preferences. Eugenics requires prioritizing certain traits, such as intelligence, health, or beauty, and accepting trade-offs, as enhancing one trait often means sacrificing others. For instance, a more intelligent brain is also a more resource-costly brain (Lieberman, 2011, pp. 222-223), and better health requires a better immune system and more DNA repair mechanisms, so that some other traits must be sacrificed. Intelligence is perhaps the most valued trait, yet it likely faces diminishing returns as success among high IQ levels depends more on other factors. Contra Fuerle, there seems to be no such evidence of diminishing returns however (Bratsberg et al., 2024; Brown et al., 2021; Haier et al., 2023, pp. 292-293; Wai, 2013, 2014).
Despite the benefits of eugenics, modern policies often promote dysgenics, such as miscegenation and welfare systems that incentivize higher birth rates among less intelligent populations. This has led to declining birth rates among high-IQ individuals and rising rates of unplanned pregnancies among lower-IQ groups, exacerbated by contraception and welfare policies. In the U.S., low-educated women have significantly higher rates of unplanned births compared to college-educated women. Welfare systems further encourage this trend by providing financial support to single mothers, disproportionately benefiting those with lower intelligence and weaker self-control.
Eugenics has been successfully applied to plants and animals for millennia, creating diverse breeds through selective breeding. However, applying eugenics to humans is controversial, as it conflicts with the egalitarian belief in genetic equality. Despite this, humans naturally practice eugenics through mate selection. Indeed, every time a person selects or rejects a person for a sexual relationship, he or she is practicing eugenics. Genetic screening is also not an uncommon practice of eugenics, as it avoids passing on harmful mutations.
The fear of eugenics stems from its potential misuse by governments, which might prioritize traits beneficial to those in power rather than individual preferences. For example, sperm banks reveal that women prioritize a mix of traits, including intelligence, health, and characteristics similar to themselves, when selecting donors. If government bureaucrats do the selecting though, the outcome would be dysgenic.
Chapter 33 – Re-Classifying the Left
Behaviors are classified as mental illnesses in psychiatric manuals when they impair normal functioning, such as working or self-care, and are biologically maladaptive by reducing reproductive success. Yet there are inconsistencies in psychiatric classifications. On one hand, homosexuality, once listed as a mental illness, was removed in 1973 due to political pressures, despite being maladaptive as it doesn’t promote allele transmission. On the other hand, conditions like schizophrenia, which also have genetic bases, remain in the manual.
Ethnocentrism, unlike homophobia, is deeply rooted in human biology. The act of helping one’s racial group is adaptive due to promoting genetic survival (Salter, 2007, p. 172). The act of mating with one’s own racial group is driven by genetic interest because it allows one to place more of his alleles in future generations by mating with people who carry more of his alleles (Salter, 2007, pp. 262-263). Ethnocentrism is innate: every normal person is programmed to pass on his or her unique set of alleles and anyone not so programmed is an accident of nature. Group cohesion is essential for survival, but yet again it relies on genetic similarity (Salter, 2007, pp. 178, 237, 267, 272, 304).
Anti-racist behaviors, by prioritizing other races, are more maladaptive than behaviors like murder, as they substantially reduce genetic fitness. Based on this logic, Fuerle concludes that anti-racism should be considered a mental illness.
Chapter 34 – Egalitarianism
The idea of human equality, particularly in its biological application, is a profound falsehood, as noted by Dr. Earnest Hooton. Having its roots in Marxism and communism, egalitarianism has evolved into a belief in genetic equality, by denying differences between populations except superficial traits like appearance. This ideology extends to genetic traits, by ignoring the evolutionary adaptations that differentiate races and sexes. Fuerle argues that if there are no genetically-induced race differences, then there should be no ethical argument against an all-white society and there should be no argument made for the integration and immigration of another race.
Feminism, a derivative of egalitarianism, asserts that men and women are genetically equal and interchangeable, dismissing biological differences in abilities like science and mathematics, including differences in brain volume and structure that are detectable at birth (Gilmore et al., 2007; Khan et al., 2024; Knickmeyer et al., 2008; Lyall et al., 2015). Perhaps due to appealing to emotions like empathy, egalitarianism obviously rejects the notion that humans, like other animals, evolve differently to adapt to their environments and compete for resources. It demands that individuals prioritize empathy for out-groups over their own genetic interests, undermining the natural group cohesion essential for survival.
Chapter 35 – Individualism
Individualism requires treating each person as an individual, not as a member of a group. For some individualists, this means that no information can be drawn from racial differences (i.e., they are trivial). The prohibition against refusing to contract with a client for a racial reason is incompatible with individualism. Civil Rights laws, which require non-discrimination in public places, are incompatible with individualism because it implies respect for each individual’s choices. Egalitarians approve of individualism when it comes to treating people according to “the content of their character,” but reject it when it is used to defend the freedom of choice, making individualism not an end in itself, but a weapon to attack racism.
If people must be treated as individuals, and their choices must be respected, then it cannot be unethical for them to act as individuals and make their own choices, even if those choices benefit them and not others. In The Virtue of Selfishness, Ayn Rand suggests that it is even a virtue to act in one’s own interest; she condemns altruism, the sacrifice of one’s own interests for the benefit of others, even if it is voluntary. Men are not things, meant to serve others, but autonomous beings with the right to live their life so as to achieve their values. Yet Rand’s philosophy seems to imply that men could either choose values that will result in the survival of their own lineage, or not, and this goes against what nature requires of her creations.
If individualism requires that individuals treat everyone based on the content of their character without regard to race (which ironically is what Rand would have advocated for, contra Fuerle), it condemns individual choice and becomes a form of collectivism, as an attempt to limit our choices to only those approved by the “Equality Police”. A person who acts against the interests of their group not only betrays their peers but also all their ancestors who sacrificed and died to allow this group to exist.
Chapter 36 – Morality
The morality of sacrifice, often promoted by egalitarians, is used to guilt the wealthy into surrendering their resources, but it contradicts evolutionary principles. Sacrifice is only adaptive if it increases the likelihood of passing on one’s alleles, making it a biological necessity rather than true altruism. A morality that demands self-sacrifice to the point of extinction is inherently flawed. Yet, a charity aiding only white people would be condemned as racist.
Egalitarianism’s principle goes against a dual morality, and this is perhaps its biggest weakness. As Fuerle cogently observed, “The existence of a group, any kind of a group, necessitates dual behavior, i.e., people in the group must behave one way towards members of the group and a different way towards outsiders, for otherwise they cannot function as a group; this suggests that at least some behavior that is immoral within a group will be moral between groups.”
Chapter 37 – Which Way Western Man?
Here, Fuerle argues that white populations, once dominant due to traits like cooperation and altruism, now face decline because these same traits have become maladaptive in a multicultural world. Every year, billions of dollars are transferred from whites to blacks, subsidizing black children. These transfers include social benefits, housing subsidies, food stamps, Medicaid, schooling subsidies, and international aid. Companies must comply with affirmative action laws and pay damages for any discrimination. Fuerle’s biggest concern is the displacement of white people through declining birthrates, immigration, interracial relationships. Fuerle singles out the European Jews, noticing that they preserved themselves by prioritizing in-group cohesion and exclusivity.
References:
Adcock, G. J., Dennis, E. S., Easteal, S., Huttley, G. A., Jermiin, L. S., Peacock, W. J., & Thorne, A. (2001). Mitochondrial DNA sequences in ancient Australians: implications for modern human origins. Proceedings of the National Academy of Sciences, 98(2), 537–542.
Ahern, F. M., Cole, R. E., Johnson, R. C., & Wong, B. (1981). Personality attributes of males and females marrying within vs. across racial/ethnic groups. Behavior Genetics, 11(3), 181–194.
Al-Zahery, N., Pala, M., Battaglia, V., Grugni, V., Hamod, M. A., Kashani, B. H., Olivieri, A., Torroni, A., Santachiara-Benerecetti, A. S., & Semino, O. (2011). In search of the genetic footprints of Sumerians: a survey of Y-chromosome and mtDNA variation in the Marsh Arabs of Iraq. BMC Evolutionary Biology, 11, 1–16.
Albalat, R., & Cañestro, C. (2016). Evolution by gene loss. Nature Reviews Genetics, 17, 379–391.
Ali, A., Lin, S. L., He, J. K., Kong, F. M., Yu, J. H., & Jiang, H. S. (2019). Climate and soils determine aboveground biomass indirectly via species diversity and stand structural complexity in tropical forests. Forest Ecology and Management, 432, 823–831.
Almarri, M. A., Haber, M., Lootah, R. A., Hallast, P., Al Turki, S., Martin, H. C., Xue, Y., & Tyler-Smith, C. (2021). The genomic history of the Middle East. Cell, 184(18), 4612–4625.
Alves Barboza, R., da Silva, E. A., Ruellas, T., & Damião, R. (2018). Anthropometric study of penile length in self-declared Brazilians regarding the color of the skin as white or black: The study of a Myth. International Journal of Impotence Research, 30(1), 43–47.
Ama, P. F.M. et al. (1986). Skeletal muscle characteristics in sedentary Black and Caucasian males. Journal of Applied Physiology, 61, 1758–1761.
Antón, S. C., Potts, R., & Aiello, L. C. (2014). Evolution of early Homo: an integrated biological perspective. Science, 345(6192), 1236828.
Antonio, M. L., Gao, Z., Moots, H. M., Lucci, M., Candilio, F., Sawyer, S., ... & Pritchard, J. K. (2019). Ancient Rome: A genetic crossroads of Europe and the Mediterranean. Science, 366(6466), 708–714.
Armstrong, T. A., Boisvert, D. L., Wells, J., Lewis, R. H., Cooke, E. M., Woeckener, M., ... & Harper, J. M. (2022). Testosterone, cortisol, and criminal behavior in men and women. Hormones and Behavior, 146, 105260.
Arnold, M. L., & Meyer, A. (2006). Natural hybridization in primates: one evolutionary mechanism. Zoology, 109(4), 261–276.
Athreya, S., & Wu, X. (2017). A multivariate assessment of the Dali hominin cranium from China: Morphological affinities and implications for Pleistocene evolution in East Asia. American Journal of Physical Anthropology, 164(4), 679-701.
Bar-Haim, Y., Ziv, T., Lamy, D., & Hodes, R. M. (2006). Nature and nurture in own-race face processing. Psychological Science, 17(2), 159–163.
Barreiro, L. B., Laval, G., Quach, H., Patin, E., & Quintana-Murci, L. (2008). Natural selection has driven population differentiation in modern humans. Nature Genetics, 40(3), 340–345.
Bastir, M., Sanz‐Prieto, D., Burgos, M. A., Pérez‐Ramos, A., Heuzé, Y., Maréchal, L., Evteev, A., Toro-Ibacache, V., & Esteban‐Ortega, F. (2024). Beyond skeletal studies: A computational analysis of nasal airway function in climate adaptation. American Journal of Biological Anthropology, 184(2), e24932.
Bates, T. C., Luciano, M., Lind, P. A., Wright, M. J., Montgomery, G. W., & Martin, N. G. (2008). Recently-derived variants of brain-size genes ASPM, MCPH1, CDK5RAP and BRCA1 not associated with general cognition, reading or language. Intelligence, 36(6), 689–693.
Baughman, H. M., Giammarco, E. A., Veselka, L., Schermer, J. A., Martin, N. G., Lynskey, M., & Vernon, P. A. (2012). A behavioral genetic study of humor styles in an Australian sample. Twin Research and Human Genetics, 15(5), 663–667.
Beach, D. (1998). Cognitive archaeology and imaginary history at Great Zimbabwe. Current Anthropology, 39(1), 47–72.
Beals, K. L., Smith, C. L., Dodd, S. M., Angel, J. L., Armstrong, E., Blumenberg, B., ... & Trinkaus, E. (1984). Brain size, cranial morphology, climate, and time machines [and comments and reply]. Current Anthropology, 25(3), 301–330.
Beaton, A. A., Rudling, N., Kissling, C., Taurines, R., & Thome, J. (2011). Digit ratio (2D: 4D), salivary testosterone, and handedness. Laterality, 16(2), 136–155.
Bednarik, R. G. (2013). African Eve: hoax or hypothesis?. Advances in Anthropology, 3(4), 216–228.
Begun, D. R. (2016). The real planet of the apes: A new story of human origins. Princeton University Press.
Beleza, S., Santos, A. M., McEvoy, B., Alves, I., Martinho, C., Cameron, E., Shriver, M. D., Parra, E. J., & Rocha, J. (2013). The timing of pigmentation lightening in Europeans. Molecular Biology and Evolution, 30(1), 24–35.
Bereczkei, T., Gyuris, P., & Weisfeld, G. E. (2004). Sexual imprinting in human mate choice. Proceedings of the Royal Society of London. Series B: Biological Sciences, 271(1544), 1129–1134.
Bereczkei, T., Hegedus, G., & Hajnal, G. (2009). Facialmetric similarities mediate mate choice: sexual imprinting on opposite-sex parents. Proceedings of the Royal Society B: Biological Sciences, 276, 91–98.
Bergström, A., Stringer, C., Hajdinjak, M., Scerri, E. M., & Skoglund, P. (2021). Origins of modern human ancestry. Nature, 590, 229–237.
Betti, L. (2017). Human Variation in Pelvic Shape and the Effects of Climate and Past Population History. The Anatomical Record, 300(4), 687–697.
Betti, L., & Manica, A. (2018). Human variation in the shape of the birth canal is significant and geographically structured. Proceedings of the Royal Society B, 285(1889), 20181807.
Bettle, R., & Rosati, A. G. (2021). Understanding Human Gaze. In T. K., Shackelford, & V. A., Weekes-Shackelford (Eds.), Encyclopedia of Evolutionary Psychological Science (pp. 8274–8277). Cham, Switzerland: Springer.
Beutler, E., & West, C. (2005). Hematologic differences between African-Americans and whites: the roles of iron deficiency and α-thalassemia on hemoglobin levels and mean corpuscular volume. Blood, 106(2), 740–745.
Böhme, M., Spassov, N., Ebner, M., Geraads, D., Hristova, L., Kirscher, U., ... & Winklhofer, M. (2017). Messinian age and savannah environment of the possible hominin Graecopithecus from Europe. PLoS One, 12(5), e0177347.
Borneman, A. R., Gianoulis, T. A., Zhang, Z. D., Yu, H., Rozowsky, J., Seringhaus, M. R., Wang, L. Y., Gerstein, M., & Snyder, M. (2007). Divergence of transcription factor binding sites across related yeast species. Science, 317(5839), 815–819.
Botero, C. A., Weissing, F. J., Wright, J., & Rubenstein, D. R. (2015). Evolutionary tipping points in the capacity to adapt to environmental change. Proceedings of the National Academy of Sciences, 112(1), 184–189.
Bratsberg, B., Rogeberg, O., & Terviö, M. (2024). Steeper at the top: cognitive ability and earnings in Finland and Norway. European Sociological Review, jcae020.
Breit, M., Scherrer, V., Tucker-Drob, E. M., & Preckel, F. (2024). The stability of cognitive abilities: A meta-analytic review of longitudinal studies. Psychological Bulletin, 150(4), 399–439.
Brown, M. I., Wai, J., & Chabris, C. F. (2021). Can you ever be too smart for your own good? Comparing linear and nonlinear effects of cognitive ability on life outcomes. Perspectives on Psychological Science, 16(6), 1337–1359.
Cabrera, V. M., Marrero, P., Abu-Amero, K. K., & Larruga, J. M. (2018). Carriers of mitochondrial DNA macrohaplogroup L3 basal lineages migrated back to Africa from Asia around 70,000 years ago. BMC Evolutionary Biology, 18, 98.
Calvin, W. H. (1991). The throwing madonna: Essays on the brain.
Campbell, M. C., & Tishkoff, S. A. (2008). African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annual Review of Genomics and Human Genetics, 9, 403–433.
Cantwell, M. F., McKenna, M. T., McCray, E., & Onorato, I. M. (1998). Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. American Journal of Respiratory and Critical Care Medicine, 157(4), 1016–1020.
Cartwright, C., Ragni, A., Hublin, J. J., & Chirchir, H. (2024). Trabecular bone volume fraction in Holocene and Late Pleistocene humans. Journal of Human Evolution, 190, 103499.
Černý, V., Pereira, L., Kujanová, M., Vašíková, A., Hájek, M., Morris, M., & Mulligan, C. J. (2009). Out of Arabia—the settlement of island Soqotra as revealed by mitochondrial and Y chromosome genetic diversity. American Journal of Physical Anthropology: The Official Publication of the American Association of Physical Anthropologists, 138(4), 439–447.
Chandrasekar, A., Kumar, S., Sreenath, J., Sarkar, B. N., Urade, B. P., Mallick, S., ... & Rao, V. R. (2009). Updating phylogeny of mitochondrial DNA macrohaplogroup m in India: dispersal of modern human in South Asian corridor. PLoS One, 4(10), e7447.
Chapais, B. (2008). Primeval Kinship: How Pair Bonding Gave Birth to Human Society. Cambridge, MA: Harvard University Press.
Charlesworth, D., & Willis, J. H. (2009). The genetics of inbreeding depression. Nature Reviews Genetics, 10, 783–796.
Cherkas, L., Hochberg, F., MacGregor, A. J., Snieder, H., & Spector, T. D. (2000). Happy families: A twin study of humour. Twin Research and Human Genetics, 3(1), 17–22.
Clavel, J., Julliard, R., & Devictor, V. (2011). Worldwide decline of specialist species: toward a global functional homogenization?. Frontiers in Ecology and the Environment, 9(4), 222–228.
Clemente, F., Unterländer, M., Dolgova, O., Amorim, C. E. G., Coroado-Santos, F., Neuenschwander, S., ... & Papageorgopoulou, C. (2021). The genomic history of the Aegean palatial civilizations. Cell, 184(10), 2565–2586.
Cochran, G., & Harpending, H. (2009). The 10,000 year explosion: How civilization accelerated human evolution. Basic Books.
Cole, C. B., Zhu, S. J., Mathieson, I., Prüfer, K., & Lunter, G. (2020). Ancient Admixture into Africa from the ancestors of non-Africans. bioRxiv.
Coon, C. S. (1962). The origin of races. New York: Knopf.
Coop, G., Pickrell, J. K., Novembre, J., Kudaravalli, S., Li, J., Absher, D., Myers, R. M., Cavalli-Sforza, L. L., Feldman, M. W., & Pritchard, J. K. (2009). The role of geography in human adaptation. PLoS Genetics, 5(6), e1000500.
Coppinger, R., & Schneider, R. (1995). Evolution of working dogs. In Serpell, J. (Ed.), The Domestic Dog: Its Evolution, Behaviour and Interactions with People (pp. 21–47). Cambridge: Cambridge University Press.
Corballis, M. C. (1991). The Lopsided Ape: Evolution of the Generative Mind. New York, NY: Oxford University Press.
Corballis, M. C. (2003). From mouth to hand: gesture, speech, and the evolution of right-handedness. Behavioral and Brain Sciences, 26(2), 199–208.
Corballis, M. C. (2009). The evolution of language. Annals of the New York Academy of Sciences, 1156, 19–43.
Crowther, A., Lucas, L., Helm, R., Horton, M., Shipton, C., Wright, H. T., ... & Boivin, N. L. (2016). Ancient crops provide first archaeological signature of the westward Austronesian expansion. Proceedings of the National Academy of Sciences, 113(24), 6635–6640.
Curnoe, D., & Thorne, A. (2003). Number of ancestral human species: a molecular perspective. Homo, 53(3), 201–224.
d’Errico, F., Backwell, L., Villa, P., Degano, I., Lucejko, J. J., Bamford, M. K., ... & Beaumont, P. B. (2012). Early evidence of San material culture represented by organic artifacts from Border Cave, South Africa. Proceedings of the National Academy of Sciences, 109(33), 13214–13219.
Daly, M., & Wilson, M. (2001). An assessment of some proposed exceptions to the phenomenon of nepotistic discrimination against stepchildren. Annales Zoologici Fennici, 38, 287–96.
Dannemann, M., & Kelso, J. (2017). The contribution of Neanderthals to phenotypic variation in modern humans. The American Journal of Human Genetics, 101(4), P578–589.
Davis, T., Hennes, E. P., & Raymond, L. (2018). Cultural evolution of normative motivations for sustainable behaviour. Nature Sustainability, 1, 218–224.
de Queiroz, K. (2007). Species concepts and species delimitation. Systematic Biology, 56(6), 879–886.
de Waal, F., & Lanting, F. (1997). Bonobo: The forgotten ape. Berkeley, CA: University of California Press.
Deary, I. J. (2014). The stability of intelligence from childhood to old age. Current Directions in Psychological Science, 23(4), 239–245.
Deary, I. J., Whalley, L. J., Lemmon, H., Crawford, J. R., & Starr, J. M. (2000). The stability of individual differences in mental ability from childhood to old age: Follow-up of the 1932 Scottish Mental Survey. Intelligence, 28(1), 49–55.
Dediu, D. (2021). Tone and genes: New cross-linguistic data and methods support the weak negative effect of the “derived” allele of ASPM on tone, but not of Microcephalin. PLoS One, 16(6), e0253546.
Dediu, D., & Levinson, S. C. (2013). On the antiquity of language: the reinterpretation of Neandertal linguistic capacities and its consequences. Frontiers in Psychology, 4, 397.
Dediu, D., & Levinson, S. C. (2018). Neanderthal language revisited: not only us. Current Opinion in Behavioral Sciences, 21, 49–55.
Deka, R., Jin, L., Shriver, M. D., Yu, L. M., DeCroo, S., Hundrieser, J., ... & Chakraborty, R. (1995). Population genetics of dinucleotide (dC-dA) n.(dG-dT) n polymorphisms in world populations. American Journal of Human Genetics, 56(2), 461–474.
den Hoed, M., Brage, S., Zhao, J. H., Westgate, K., Nessa, A., Ekelund, U., ... & Loos, R. J. (2013). Heritability of objectively assessed daily physical activity and sedentary behavior. The American Journal of Clinical Nutrition, 98(5), 1317–1325.
Dennell, R., & Roebroeks, W. (2005). An Asian perspective on early human dispersal from Africa. Nature, 438, 1099–1104.
DeSilva, J., Fannin, L., Cheney, I., Claxton, A., Ilieş, I., Kittelberger, J., Stibel, J., & Traniello, J. (2023). Human brains have shrunk: the questions are when and why. Frontiers in Ecology and Evolution, 11, 1191274.
Ding, Y. C., Chi, H. C., Grady, D. L., Morishima, A., Kidd, J. R., Kidd, K. K., ... & Moyzis, R. K. (2002). Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proceedings of the National Academy of Sciences, 99(1), 309–314.
Dobson-Stone, C., Gatt, J. M., Kuan, S. A., Grieve, S. M., Gordon, E., Williams, L. M., & Schofield, P. R. (2007). Investigation of MCPH1 G37995C and ASPM A44871G polymorphisms and brain size in a healthy cohort. Neuroimage, 37(2), 394–400.
Doddi, N. M., & Eccles, R. (2011). The relationship between nasal index and nasal airway resistance, and response to a topical decongestant. Rhinology, 49(5), 583–586.
Dong, Z., Wang, Y., Li, C., Li, L., & Men, X. (2021). Mitochondrial DNA as a molecular marker in insect ecology: current status and future prospects. Annals of the Entomological Society of America, 114(4), 470–476.
Drake, N. A., Blench, R. M., Armitage, S. J., Bristow, C. S., & White, K. H. (2011). Ancient watercourses and biogeography of the Sahara explain the peopling of the desert. Proceedings of the National Academy of Sciences, 108(2), 458–462.
Drummond-Clarke, R. C., Kivell, T. L., Sarringhaus, L., Stewart, F. A., Humle, T., & Piel, A. K. (2022). Wild chimpanzee behavior suggests that a savanna-mosaic habitat did not support the emergence of hominin terrestrial bipedalism. Science Advances, 8(50), eadd9752.
Dunbar, R. I. (1998). The Social Brain Hypothesis. Evolutionary Anthropology: Issues, News, and Review, 6(5), 178–190.
Dunbar, R. I. (2009). The social brain hypothesis and its implications for social evolution. Annals of Human Biology, 36(5), 562–572.
Dunsworth, H. M. (2010). Origin of the genus Homo. Evolution: Education and Outreach, 3, 353–366.
Dunsworth, H. M., Warrener, A. G., Deacon, T., Ellison, P. T., & Pontzer, H. (2012). Metabolic hypothesis for human altriciality. Proceedings of the National Academy of Sciences, 109(38), 15212–15216.
Dyola, N., Sigdel, S. R., Liang, E., Babst, F., Camarero, J. J., Aryal, S., ... & Peñuelas, J. (2022). Species richness is a strong driver of forest biomass along broad bioclimatic gradients in the Himalayas. Ecosphere, 13(6), e4107.
Edmands, S. (2007). Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Molecular Ecology, 16(3), 463–475.
Eiberg, H., Troelsen, J., Nielsen, M., Mikkelsen, A., Mengel-From, J., Kjaer, K. W., & Hansen, L. (2008). Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Human Genetics, 123, 177–187.
Ekblom, R. (2000). Inbreeding avoidance through mate choice.
Ellis, E. C., Beusen, A. H., & Klein Goldewijk, K. (2020). Anthropogenic biomes: 10,000 BCE to 2015 CE. Land, 9(5), 129.
Estes, S., & Arnold, S. J. (2007). Resolving the paradox of stasis: models with stabilizing selection explain evolutionary divergence on all timescales. The American Naturalist, 169(2), 227–244.
Eswaran, V., Harpending, H., & Rogers, A. R. (2005). Genomics refutes an exclusively African origin of humans. Journal of Human Evolution, 49(1), 1–18.
Fadhlaoui-Zid, K., Haber, M., Martínez-Cruz, B., Zalloua, P., Benammar Elgaaied, A., & Comas, D. (2013). Genome-wide and paternal diversity reveal a recent origin of human populations in North Africa. PLoS One, 8(11), e80293.
Fan, S., Hansen, M. E., Lo, Y., & Tishkoff, S. A. (2016). Going global by adapting local: A review of recent human adaptation. Science, 354(6308), 54–59.
Fan, S., Kelly, D. E., Beltrame, M. H., Hansen, M. E., Mallick, S., Ranciaro, A., ... & Tishkoff, S. A. (2019). African evolutionary history inferred from whole genome sequence data of 44 indigenous African populations. Genome Biology, 20, 82.
Fan, S., Spence, J. P., Feng, Y., Hansen, M. E., Terhorst, J., Beltrame, M. H., ... & Tishkoff, S. A. (2023). Whole-genome sequencing reveals a complex African population demographic history and signatures of local adaptation. Cell, 186(5), 923–939.
Fernandes, V., Brucato, N., Ferreira, J. C., Pedro, N., Cavadas, B., Ricaut, F. X., Alshamali, F., & Pereira, L. (2019). Genome-wide characterization of Arabian Peninsula populations: shedding light on the history of a fundamental bridge between continents. Molecular Biology and Evolution, 36(3), 575–586.
Fernandes, V., Triska, P., Pereira, J. B., Alshamali, F., Rito, T., Machado, A., Fajkošová, Z., Cavadas, B., Černý, V., Soares, P., Richards, M. B., & Pereira, L. (2015). Genetic stratigraphy of key demographic events in Arabia. PLoS One, 10(3), e0118625.
Ferreira, T., & Rodriguez, S. (2024). Mitochondrial DNA: Inherent complexities relevant to genetic analyses. Genes, 15(5), 617.
Ferrer-Admetlla, A., Bosch, E., Sikora, M., Marques-Bonet, T., Ramírez-Soriano, A., Muntasell, A., ... & Casals, F. (2008). Balancing selection is the main force shaping the evolution of innate immunity genes. The Journal of Immunology, 181(2), 1315–1322.
Fleagle, J. G., Assefa, Z., Brown, F. H., & Shea, J. J. (2008). Paleoanthropology of the Kibish Formation, southern Ethiopia: introduction. Journal of Human Evolution, 55(3), 360–365.
Foster, F., & Collard, M. (2013). A reassessment of Bergmann's rule in modern humans. PLoS One, 8(8), e72269.
Francis, G., & Kirkegaard, E. O. W. (2023). Racial Differences in Sexuality: A Sex Worker Survey. Mankind Quarterly, 63(4), 675–698.
Frankham, R. (2005). Genetics and extinction. Biological Conservation, 126(2), 131–140.
Frenkel-Pinter, M., Petrov, A. S., Matange, K., Travisano, M., Glass, J. B., & Williams, L. D. (2022). Adaptation and exaptation: from small molecules to feathers. Journal of Molecular Evolution, 90, 166–175.
Frost, P. (1990). Fair women, dark men: The forgotten roots of colour prejudice. History of European Ideas, 12(5), 669–679.
Frost, P. (2008). The spread of alphabetical writing may have favored the latest variant of the ASPM gene. Medical Hypotheses, 70(1), 17–20.
Frost, P. (2014a). The puzzle of European hair, eye, and skin color. Advances in Anthropology, 4(2), 78–88.
Frost, P. (2014b). L.L. Cavalli-Sforza: A bird in a gilded cage. Open Behavioral Genetics.
Frost, P. (2019). The Original Industrial Revolution. Did Cold Winters Select for Cognitive Ability?. Psych, 1(1), 166–181.
Fu, Q., Mittnik, A., Johnson, P. L., Bos, K., Lari, M., Bollongino, R., ... & Krause, J. (2013). A revised timescale for human evolution based on ancient mitochondrial genomes. Current Biology, 23(7), 553–559.
Fuerst, J. (2015). The Nature of Race: the Genealogy of the Concept and the Biological Construct’s Contemporaneous Utility. Open Behavioral Genetics.
Fuku, N., Kumagai, H., & Ahmetov, I. I. (2019). Genetics of muscle fiber composition. In D., Barh & I. I., Ahmetov (Eds.), Sports, Exercise, and Nutritional Genomics: Current Status and Future Directions (pp. 295–314). Academic Press.
Funk, D. J., & Omland, K. E. (2003). Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annual Review of Ecology, Evolution, and Systematics, 34, 397–423.
Fuss, J., Spassov, N., Begun, D. R., & Böhme, M. (2017). Potential hominin affinities of Graecopithecus from the Late Miocene of Europe. PLoS One, 12(5), e0177127.
Gao, J., Davis, L. K., Hart, A. B., Sanchez-Roige, S., Han, L., Cacioppo, J. T., & Palmer, A. A. (2017). Genome-wide association study of loneliness demonstrates a role for common variation. Neuropsychopharmacology, 42, 811–821.
Gao, X., Peng, F., Fu, Q., & Li, F. (2017). New progress in understanding the origins of modern humans in China. Science China Earth Sciences, 60, 2160–2170.
Ge, J., Xing, S., Grün, R., Deng, C., Jiang, Y., Jiang, T., ... & Shao, Q. (2024). New Late Pleistocene age for the Homo sapiens skeleton from Liujiang southern China. Nature Communications, 15, 3611.
Getahun, D., Ananth, C. V., Selvam, N., & Demissie, K. (2005). Adverse perinatal outcomes among interracial couples in the United States. Obstetrics & Gynecology, 106(1), 81–88.
Gichoya, J. W., Banerjee, I., Bhimireddy, A. R., Burns, J. L., Celi, L. A., Chen, L. C., ... & Zhang, H. (2022). AI recognition of patient race in medical imaging: A modelling study. Lancet Digital Health 4(6), e406–e414.
Gielen, M., Westerterp-Plantenga, M. S., Bouwman, F. G., Joosen, A. M. C. P., Vlietinck, R., Derom, C., ... & Westerterp, K. R. (2014). Heritability and genetic etiology of habitual physical activity: a twin study with objective measures. Genes & Nutrition, 9, 1–12.
Gifford-Gonzalez, D. (2000). Animal disease challenges to the emergence of pastoralism in sub-Saharan Africa. African Archaeological Review, 17, 95–139.
Gignac, G. E., & Bates, T. C. (2017). Brain volume and intelligence: The moderating role of intelligence measurement quality. Intelligence, 64, 18–29.
Gilmore, J. H., Lin, W., Prastawa, M. W., Looney, C. B., Vetsa, Y. S. K., Knickmeyer, R. C., Evans, D. D., Smith, J. K., Hamer, R. M., Lieberman, J. A., & Gerig, G. (2007). Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. Journal of Neuroscience, 27(6), 1255–1260.
Glémin, S. (2003). How are deleterious mutations purged? Drift versus nonrandom mating. Evolution, 57(12), 2678–2687.
Gomez, F., Hirbo, J., & Tishkoff, S. A. (2014). Genetic variation and adaptation in Africa: implications for human evolution and disease. Cold Spring Harbor Perspectives in Biology, 6(7), a008524.
Gomez-Mestre, I., & Jovani, R. (2013). A heuristic model on the role of plasticity in adaptive evolution: plasticity increases adaptation, population viability and genetic variation. Proceedings of the Royal Society B: Biological Sciences, 280(1771), 20131869.
Gómez-Robles, A., Nicolaou, C., Smaers, J. B., & Sherwood, C. C. (2024). The evolution of human altriciality and brain development in comparative context. Nature Ecology & Evolution, 8, 133–146.
Gonzaga, G. C., Haselton, M. G., Smurda, J., Davies, M. s., & Poore, J. C. (2008). Love, desire, and the suppression of thoughts of romantic alternatives. Evolution and Human Behavior, 29(2), 119–126.
Gonzalez, A. M., Brehm, A., Perez, J. A., Maca‐Meyer, N., Flores, C., & Cabrera, V. M. (2003). Mitochondrial DNA affinities at the Atlantic fringe of Europe. American Journal of Physical Anthropology: The Official Publication of the American Association of Physical Anthropologists, 120(4), 391–404.
González, A. M., Larruga, J. M., Abu-Amero, K. K., Shi, Y., Pestano, J., & Cabrera, V. M. (2007). Mitochondrial lineage M1 traces an early human backflow to Africa. BMC Genomics, 8, 223.
Gould, S. J., & Vrba, E. S. (1982). Exaptation—a missing term in the science of form. Paleobiology, 8(1), 4–15.
Gowlett, J. A. (2016). The discovery of fire by humans: a long and convoluted process. Philosophical Transactions of the Royal Society B: Biological Sciences, 371, 20150164.
Grabowski, M., Kopperud, B. T., Tsuboi, M., & Hansen, T. F. (2023). Both diet and sociality affect primate brain-size evolution. Systematic Biology, 72(2), 404–418.
Graur, D., Zheng, Y., Price, N., Azevedo, R. B., Zufall, R. A., & Elhaik, E. (2013). On the immortality of television sets: “function” in the human genome according to the evolution-free gospel of ENCODE. Genome Biology and Evolution, 5(3), 578–590.
Grehan, J. R. (2006). Mona Lisa smile: the morphological enigma of human and great ape evolution. The Anatomical Record Part B: The New Anatomist: An Official Publication of the American Association of Anatomists, 289(4), 139–157.
Grunstra, N. D. S., Betti, L., Fischer, B., Haeusler, M., Pavlicev, M., Stansfield, E., ... & Mitteroecker, P. (2023). There is an obstetrical dilemma: Misconceptions about the evolution of human childbirth and pelvic form. American Journal of Biological Anthropology, 181(4), 535–544.
Guidot, D. M., Wood, M., Poehlein, E., Palmer, S., & McElroy, L. (2025). Racial disparities in lung function by pulmonary function testing among lung transplant candidates and race-specific reference equations. JHLT Open, 8, 100252.
Guillén, A. K. Z., Barrett, G. M., & Takenaka, O. (2005). Genetic diversity among African great apes based on mitochondrial DNA sequences. Biodiversity & Conservation, 14, 2221–2233.
Guo, G., Fu, Y., Lee, H., Cai, T., Mullan Harris, K., & Li, Y. (2014). Genetic bio-ancestry and social construction of racial classification in social surveys in the contemporary United States. Demography, 51(1), 141–172.
Habel, J. C., & Schmitt, T. (2012). The burden of genetic diversity. Biological Conservation, 147(1), 270–274.
Haber, M., Mezzavilla, M., Bergström, A., Prado-Martinez, J., Hallast, P., Saif-Ali, R., ... & Tyler-Smith, C. (2016). Chad genetic diversity reveals an African history marked by multiple Holocene Eurasian migrations. The American Journal of Human Genetics, 99(6), 1316–1324.
Haeusler, M., Grunstra, N. D., Martin, R. D., Krenn, V. A., Fornai, C., & Webb, N. M. (2021). The obstetrical dilemma hypothesis: there’s life in the old dog yet. Biological Reviews, 96(5), 2031–2057.
Hagelberg, E. (2003). Recombination or mutation rate heterogeneity? Implications for Mitochondrial Eve. Trends in Genetics, 19(2), 84–90.
Haier, R. J., Colom, R., & Hunt, E. B. (2023). The science of human intelligence. Cambridge University Press.
Hammer, M. F., Woerner, A. E., Mendez, F. L., Watkins, J. C., & Wall, J. D. (2011). Genetic evidence for archaic admixture in Africa. Proceedings of the National Academy of Sciences, 108(37), 15123–15128.
Harcourt-Smith, W. E. (2015). Origin of Bipedal Locomotion. In W., Henke, & I., Tattersall (Eds.), Handbook of Paleoanthropology (pp. 1919–1959). Berlin: Springer.
Harding, R. M., Healy, E., Ray, A. J., Ellis, N. S., Flanagan, N., Todd, C., ... & Rees, J. L. (2000). Evidence for variable selective pressures at MC1R. The American Journal of Human Genetics, 66(4), 1351–1361.
Harris, K., & Nielsen, R. (2016). The genetic cost of Neanderthal introgression. Genetics, 203(2), 881–891.
Hawks, J., Wang, E. T., Cochran, G. M., Harpending, H. C., & Moyzis, R. K. (2007). Recent acceleration of human adaptive evolution. Proceedings of the National Academy of Sciences, 104(52), 20753–20758.
Heard, E., & Martienssen, R. A. (2014). Transgenerational epigenetic inheritance: myths and mechanisms. Cell, 157(1), 95–109.
Hedrick, P. W., & Garcia-Dorado, A. (2016). Understanding inbreeding depression, purging, and genetic rescue. Trends in Ecology & Evolution, 31(12), 940–952.
Heffernan, M. E., Chong, J. Y., & Fraley, R. C. (2019). Are people attracted to others who resemble their opposite-sex parents? An examination of mate preferences and parental ethnicity among biracial individuals. Social Psychological and Personality Science, 10(7), 856–863.
Hellenthal, G., Busby, G. B., Band, G., Wilson, J. F., Capelli, C., Falush, D., & Myers, S. (2014). A genetic atlas of human admixture history. Science, 343(6172), 747–751.
Henn, B. M., Botigué, L. R., Gravel, S., Wang, W., Brisbin, A., Byrnes, J. K., Fadhlaoui-Zid, K., Zalloua, P. A., Moreno-Estrada, A., Bertranpetit, J., Bustamante, C. D., & Comas, D. (2012a). Genomic ancestry of North Africans supports back-to-Africa migrations. PLoS Genetics, 8(1), e1002397.
Henn, B. M., Cavalli-Sforza, L. L., & Feldman, M. W. (2012b). The great human expansion. Proceedings of the National Academy of Sciences, 109(44), 17758-17764.
Henn, B. M., Gignoux, C. R., Feldman, M. W., & Mountain, J. L. (2009). Characterizing the time dependency of human mitochondrial DNA mutation rate estimates. Molecular Biology and Evolution, 26(1), 217–230.
Henrich, J., & Muthukrishna, M. (2021). The origins and psychology of human cooperation. Annual Review of Psychology, 72, 207–240.
Henshilwood, C. S., d’Errico, F., Yates, R., Jacobs, Z., Tribolo, C., Duller, G. A., ... & Wintle, A. G. (2002). Emergence of modern human behavior: Middle Stone Age engravings from South Africa. Science, 295, 1278.
Herndon, J. G. (2010). The grandmother effect: implications for studies on aging and cognition. Gerontology, 56(1), 73–79.
Hershkovitz, I., Weber, G. W., Quam, R., Duval, M., Grün, R., Kinsley, L., ... & Weinstein-Evron, M. (2018). The earliest modern humans outside Africa. Science, 359(6374), 456–459.
Heupink, T. H., Subramanian, S., Wright, J. L., Endicott, P., Westaway, M. C., Huynen, L., ... & Lambert, D. M. (2016). Ancient mtDNA sequences from the First Australians revisited. Proceedings of the National Academy of Sciences, 113(25), 6892–6897.
Heyes, C. (2012). New thinking: the evolution of human cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 367(1599), 2091–2096.
Hlubik, S., Berna, F., Feibel, C., Braun, D., & Harris, J. W. (2017). Researching the nature of fire at 1.5 Mya on the site of FxJj20 AB, Koobi Fora, Kenya, using high-resolution spatial analysis and FTIR spectrometry. Current Anthropology, 58(S16), S243–S257.
Hlusko, L. J., Carlson, J. P., Chaplin, G., Elias, S. A., Hoffecker, J. F., Huffman, M., ... & Scott, G. R. (2018). Environmental selection during the last ice age on the mother-to-infant transmission of vitamin D and fatty acids through breast milk. Proceedings of the National Academy of Sciences, 115(19), E4426–E4432.
Hobolth, A., Christensen, O. F., Mailund, T., & Schierup, M. H. (2007). Genomic relationships and speciation times of human, chimpanzee, and gorilla inferred from a coalescent hidden Markov model. PLoS Genetics, 3(2), e7.
Hodgson, J. A., Mulligan, C. J., Al-Meeri, A., & Raaum, R. L. (2014a). Early back-to-Africa migration into the Horn of Africa. PLoS Genetics, 10(6), e1004393.
Hodgson, J. A., Pickrell, J. K., Pearson, L. N., Quillen, E. E., Prista, A., Rocha, J., Soodyall, H., Shriver, M. D., & Perry, G. H. (2014b). Natural selection for the Duffy-null allele in the recently admixed people of Madagascar. Proceedings of the Royal Society B: Biological Sciences, 281(1789), 20140930.
Holt, R. D. (2009). Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proceedings of the National Academy of Sciences, 106(supplement_2), 19659–19665.
Hou, B., & Wang, Y. (2021). Westermarck effect and imprinting. In T. K., Shackelford, & V. A., Weekes-Shackelford (Eds.), Encyclopedia of Evolutionary Psychological Science (pp. 8496–8498). Cham, Switzerland: Springer.
Hu, M. (2024). The Black-White SAT Score Gap Increases with Education Level. Mankind Quarterly, 64(4), 662–689.
Hu, M. (2025). Mixed evidence for Lynn’s developmental theory of sex differences using aptitude tests. OpenPsych. Under review.
Huang, S. (2025a). Examining models of modern human origins through the analysis of 43 fully sequenced human Y chromosomes. bioRxiv.
Huang, S. (2025). The Maximum Genetic Diversity Theory: A Comprehensive Framework for Understanding Evolutionary Processes. BioCosmos, 5(1), 41–61.
Hublin, J. J., Ben-Ncer, A., Bailey, S. E., Freidline, S. E., Neubauer, S., Skinner, M. M., ... & Gunz, P. (2017). New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature, 546(7657), 289–292.
Hughes, J. F., Skaletsky, H., Pyntikova, T., Graves, T. A., Van Daalen, S. K., Minx, P. J., ... & Page, D. C. (2010). Chimpanzee and human Y chromosomes are remarkably divergent in structure and gene content. Nature, 463(7280), 536–539.
Hur, Y. M., Bouchard Jr, T. J., & Lykken, D. T. (1998). Genetic and environmental influence on morningness–eveningness. Personality and Individual Differences, 25(5), 917–925.
Iasi, L. N., Chintalapati, M., Skov, L., Mesa, A. B., Hajdinjak, M., Peter, B. M., & Moorjani, P. (2024). Neanderthal ancestry through time: Insights from genomes of ancient and present-day humans. Science, 386(6727), eadq3010.
Isler, K., & Van Schaik, C. P. (2006). Metabolic costs of brain size evolution. Biology Letters, 2(4), 557–560.
Jablonski, N. G. (2021). The evolution of human skin pigmentation involved the interactions of genetic, environmental, and cultural variables. Pigment Cell & Melanoma Research, 34(4), 707–729.
Jablonski, N. G., & Chaplin, G. (2000). The evolution of human skin coloration. Journal of Human Evolution, 39(1), 57–106.
Jablonski, N. G., & Chaplin, G. (2010). Human skin pigmentation as an adaptation to UV radiation. Proceedings of the National Academy of Sciences, 107(supplement_2), 8962–8968.
Jansen, A. G., Mous, S. E., White, T., Posthuma, D., & Polderman, T. J. (2015). What twin studies tell us about the heritability of brain development, morphology, and function: a review. Neuropsychology Review, 25, 27–46.
Jeanson, N. T. (2016). On the Origin of Human Mitochondrial DNA Differences, New Generation Time Data Both Suggest a Unified Young-Earth Creation Model and Challenge the Evolutionary Out-of-Africa Model. Answers Research Journal, 9, 123–130.
Jensen, A. R. (1973). Educability and group differences. New York: Harper & Row.
Jensen, A. R. (1998). The g factor: The science of mental ability. Westport, CT: Prager.
Jeon, J., & Buss, D. M. (2007). Altruism towards cousins. Proceedings of the Royal Society B: Biological Sciences, 274, 1181–1187.
Jeong, C., Nakagome, S., & Di Rienzo, A. (2016). Deep history of East Asian populations revealed through genetic analysis of the Ainu. Genetics, 202(1), 261–272.
Jetz, W., & Fine, P. V. (2012). Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biology, 10(3), e1001292.
Jinam, T. A., Phipps, M. E., Aghakhanian, F., Majumder, P. P., Datar, F., Stoneking, M., ... & Saitou, N. (2017). Discerning the origins of the Negritos, first Sundaland people: deep divergence and archaic admixture. Genome Biology and Evolution, 9(8), 2013–2022.
Juric, I., Aeschbacher, S., & Coop, G. (2016). The strength of selection against Neanderthal introgression. PLoS Genetics, 12(11), e1006340.
Kamberov, Y. G., Wang, S., Tan, J., Gerbault, P., Wark, A., Tan, L., ... & Sabeti, P. C. (2013). Modeling recent human evolution in mice by expression of a selected EDAR variant. Cell, 152(4), 691–702.
Katz, D. C., Grote, M. N., & Weaver, T. D. (2017). Changes in human skull morphology across the agricultural transition are consistent with softer diets in preindustrial farming groups. Proceedings of the National Academy of Sciences, 114(34), 9050–9055.
Keller, L. F., & Waller, D. M. (2002). Inbreeding effects in wild populations. Trends in Ecology & Evolution, 17(5), 230–241.
Kelly, D. J., Quinn, P. C., Slater, A. M., Lee, K., Gibson, A., Smith, M., Liezhong, G., Pascalis, O. (2005). Three-month-olds, but not newborns, prefer own-race faces. Developmental Science, 8(6), F31–F36.
Khan, Y. T., Tsompanidis, A., Radecki, M. A., Dorfschmidt, L., Austin, T., Suckling, J., ... & Baron-Cohen, S. (2024). Sex Differences in Human Brain Structure at Birth. Biology of Sex Differences, 15, 81.
Kirk, M. (2024). Genetic drift and founder effects: Implications for population genetics, conservation, and human health. Genetics and Molecular Research, 23(2), gmr34057.
Kirkegaard, E. O. W. (2021). Genetic ancestry and social race are nearly interchangeable. OpenPsych.
Kirkegaard, E. O. W., & Carl, N. (2022). Smart Fraction Theory: A Comprehensive Re-evaluation. Comparative Sociology, 21(6), 677–699.
Kirkegaard, E. O. W., & Fuerst, J. G. R. (2023). A Multimodal MRI-based Predictor of Intelligence and Its Relation to Race/Ethnicity. Mankind Quarterly, 63(3), 374–397.
Kivell, T. L., & Schmitt, D. (2009). Independent evolution of knuckle-walking in African apes shows that humans did not evolve from a knuckle-walking ancestor. Proceedings of the National Academy of Sciences, 106(34), 14241–14246.
Kassen, R. (2002). The experimental evolution of specialists, generalists, and the maintenance of diversity. Journal of Evolutionary Biology, 15(2), 173–190.
Klein, J., & Takahata, N. (2002). Where do we come from?: The molecular evidence for human descent. Berlin: Springer.
Knickmeyer, R. C., Gouttard, S., Kang, C., Evans, D., Wilber, K., Smith, J. K., ... & Gilmore, J. H. (2008). A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience, 28(47), 12176–12182.
Krems, J. A., Claessens, S., Fales, M. R., Campenni, M., Haselton, M. G., & Aktipis, A. (2021). An agent-based model of the female rivalry hypothesis for concealed ovulation in humans. Nature Human Behaviour, 5(6), 726–735.
Krupp, D. B., Debruine, L. M., & Barclay, P. (2008). A cue of kinship promotes cooperation for the public good. Evolution and Human Behavior, 29(1), 49–55.
Krupp, D. B., DeBruine, L. M., & Jones, B.C. (2011). Cooperation and conflict in the light of kin recognition systems. In C. A., Salmon, & T. K., Shackelford (Eds.), The Oxford handbook of evolutionary family psychology (pp. 345–362). New York, NY: Oxford University Press.
Kuliukas, A. V. (2002). Wading for food the driving force of the evolution of bipedalism?. Nutrition and Health, 16(4), 267–289.
Kuliukas, A. V. (2012). Langdon’s Critique of the Aquatic Ape Hypothesis: It’s Final Refutation, or Just Another Misunderstanding?. In M., Vaneechoutte, A. V., Kuliukas, & M., Verhaegen (Eds.), Was Man More Aquatic in the Past? Fifty Years After Alister Hardy: Waterside Hypotheses of Human Evolution (pp. 213–225). Bentham Science Publishers.
Kuliukas, A. V. (2014). Removing the “hermetic seal” from the aquatic ape hypothesis: Waterside hypotheses of human evolution. Advances in Anthropology, 4, 164–167.
Kurki, H. K. (2013). Bony pelvic canal size and shape in relation to body proportionality in humans. American Journal of Physical Anthropology, 151(1), 88–101.
Kwiatkowski, D. P. (2005). How malaria has affected the human genome and what human genetics can teach us about malaria. The American Journal of Human Genetics, 77(2), 171–192.
Laland, K., Matthews, B., & Feldman, M. W. (2016). An introduction to niche construction theory. Evolutionary Ecology, 30, 191–202.
Laland, K. N., Uller, T., Feldman, M. W., Sterelny, K., Müller, G. B., Moczek, A., ... & Odling-Smee, J. (2015). The extended evolutionary synthesis: its structure, assumptions and predictions. Proceedings of the Royal Society B: Biological Sciences, 282, 20151019.
Lao, O., Lu, T. T., Nothnagel, M., Junge, O., Freitag-Wolf, S., Caliebe, A., ... & Kayser, M. (2008). Correlation between genetic and geographic structure in Europe. Current Biology, 18(16), P1241–1248.
Lane, C. S., Chorn, B. T., & Johnson, T. C. (2013). Ash from the Toba supereruption in Lake Malawi shows no volcanic winter in East Africa at 75 ka. Proceedings of the National Academy of Sciences, 110(20), 8025–8029.
Lazaridis, I., Alpaslan-Roodenberg, S., Acar, A., Açıkkol, A., Agelarakis, A., Aghikyan, L., ... & Ruka, R. (2022). The genetic history of the Southern Arc: A bridge between West Asia and Europe. Science, 377(6609), eabm4247.
Lazaridis, I., Mittnik, A., Patterson, N., Mallick, S., Rohland, N., Pfrengle, S., ... & Stamatoyannopoulos, G. (2017). Genetic origins of the Minoans and Mycenaeans. Nature, 548(7666), 214–218.
Ledogar, J. A., Dechow, P. C., Wang, Q., Gharpure, P. H., Gordon, A. D., Baab, K. L., ... & Strait, D. S. (2016). Human feeding biomechanics: performance, variation, and functional constraints. PeerJ, 4, e2242.
Leinonen, T., McCairns, R. S., O’hara, R. B., & Merilä, J. (2013). Q ST–F ST comparisons: evolutionary and ecological insights from genomic heterogeneity. Nature Reviews Genetics, 14(3), 179–190.
Lewin, R. (1993). The origin of modern humans. New York: Scientific American Library.
Li, S., Jovelin, R., Yoshiga, T., Tanaka, R., & Cutter, A. D. (2014). Specialist versus generalist life histories and nucleotide diversity in Caenorhabditis nematodes. Proceedings of the Royal Society B: Biological Sciences, 281, 20132858.
Li, Y. C., Gao, Z. L., Liu, K. J., Tian, J. Y., Yang, B. Y., Rahman, Z. U., ... & Kong, Q. P. (2023). Mitogenome evidence shows two radiation events and dispersals of matrilineal ancestry from northern coastal China to the Americas and Japan. Cell Reports, 42(5).
Lieberman, D. E. (2011). The evolution of the human head. Cambridge, MA: Belknap Press.
Lin, M., Siford, R. L., Martin, A. R., Nakagome, S., Möller, M., Hoal, E. G., Bustamante, C. D., Gignoux, C. R., & Henn, B. M. (2018). Rapid evolution of a skin-lightening allele in southern African KhoeSan. Proceedings of the National Academy of Sciences, 115(52), 13324–13329.
Littlefield, C. H., & Rushton, J. P. (1986). When a child dies: The sociobiology of bereavement. Journal of Personality and Social Psychology, 51(4), 797–802.
Liu, W., & Wu, X. (2022). Morphological diversities of the late-Middle and Late Pleistocene human fossils in China. Acta Anthropologica Sinica, 41(4), 563–575.
Long, J. C. (2009). Update to Long and Kittles’s “human genetic diversity and the nonexistence of biological races” (2003): fixation on an index. Human Biology, 81(5), 799–803.
Longman, D. P., Wells, J. C., & Stock, J. T. (2020). Human athletic paleobiology; using sport as a model to investigate human evolutionary adaptation. American Journal of Physical Anthropology, 171(S70), 42–59.
Lukas, D., & Clutton-Brock, T. H. (2013). The evolution of social monogamy in mammals. Science, 341(6145), 526–530.
Luo, S., Valencia, C. A., Zhang, J., Lee, N. C., Slone, J., Gui, B., ... & Huang, T. (2018). Biparental inheritance of mitochondrial DNA in humans. Proceedings of the National Academy of Sciences, 115(51), 13039–13044.
Lüpold, S., de Boer, R. A., Evans, J. P., Tomkins, J. L., & Fitzpatrick, J. L. (2020). How sperm competition shapes the evolution of testes and sperm: a meta-analysis. Philosophical Transactions of the Royal Society B, 375(1813), 20200064.
Luzzatto, L. (2012). Sickle cell anaemia and malaria. Mediterranean Journal of Hematology and Infectious Diseases, 4(1), e2012065.
Lyall, A. E., Shi, F., Geng, X., Woolson, S., Li, G., Wang, L., Hamer, R. M., Shen, D., & Gilmore, J. H. (2015). Dynamic development of regional cortical thickness and surface area in early childhood. Cerebral Cortex, 25(8), 2204–2212.
Lynch, M. (2016). Mutation and human exceptionalism: our future genetic load. Genetics, 202(3), 869–875.
Lynn, R. (2006). Race differences in intelligence: an evolutionary analysis. Washington Summit Publishers.
Lynn, R. (2019). Race differences in psychopathic personality: An evolutionary analysis. Washington Summit Publishing.
Lynn, R., & Becker, D. (2019). The intelligence of nations. London, UK: Ulster Institute for Social Research.
Maddux, S. D., Butaric, L. N., Yokley, T. R., & Franciscus, R. G. (2017). Ecogeographic variation across morphofunctional units of the human nose. American Journal of Physical Anthropology, 162(1), 103–119.
Madison, G. (2021). Parental Effort Versus Mating Effort. In T. K., Shackelford, & V. A., Weekes-Shackelford (Eds.), Encyclopedia of Evolutionary Psychological Science (pp. 5701–5704). Cham, Switzerland: Springer.
Magadum, S., Banerjee, U., Murugan, P., Gangapur, D., & Ravikesavan, R. (2013). Gene duplication as a major force in evolution. Journal of Genetics, 92, 155–161.
Magalhães, J. R. (1997). Africans, Indians, and Slavery in Portugal. Portuguese Studies, 13, 143–151.
Malaspinas, A. S., Westaway, M. C., Muller, C., Sousa, V. C., Lao, O., Alves, I., ... & Willerslev, E. (2016). A genomic history of Aboriginal Australia. Nature, 538, 207–214.
Manning, J. T., & Fink, B. (2021). Digit Ratio. In T. K., Shackelford, & V. A., Weekes-Shackelford (Eds.), Encyclopedia of Evolutionary Psychological Science (pp. 2009–2016). Cham, Switzerland: Springer.
Manning, J. T., Fink, B., & Trivers, R. (2021). The Biology of Human Gender. In T. K., Shackelford, & V. A., Weekes-Shackelford (Eds.), Encyclopedia of Evolutionary Psychological Science (pp. 586–589). Cham, Switzerland: Springer.
Manning, J. T., Churchill, A. J., & Peters, M. (2007). The effects of sex, ethnicity, and sexual orientation on self-measured digit ratio (2D: 4D). Archives of Sexual Behavior, 36, 223–233.
Manning, J. T., Stewart, A., Bundred, P. E., & Trivers, R. (2004). Sex and ethnic differences in 2nd to 4th digit ratio of children. Early Human Development, 80(2), 161–168.
Mansfield, F. A., & Vaneechoutte, M. (2024). Current evidence indicates a Eurasian origin for the Last Common Ancestor of African apes and humans, and supports a new hypothesis suggesting that the Zanclean Megaflood (5.3 Ma) may have played a role in the ultimate divergence of Pan and Homo. Ideas in Ecology and Evolution, 17, 1–21.
Manuck, T. A. (2017). Racial and ethnic differences in preterm birth: a complex, multifactorial problem. Seminars in Perinatology, 41(8), 511–518.
Mao, X., Zhang, H., Qiao, S., Liu, Y., Chang, F., Xie, P., ... & Fu, Q. (2021). The deep population history of northern East Asia from the Late Pleistocene to the Holocene. Cell, 184(12), 3256–3266.
Marcinkowska, U. M., & Rantala, M. J. (2012). Sexual imprinting on facial traits of opposite-sex parents in humans. Evolutionary Psychology, 10(3), 621–630.
McBrearty, S., & Brooks, A. S. (2000). The revolution that wasn’t: a new interpretation of the origin of modern human behavior. Journal of Human Evolution, 39(5), 453–563.
McGrath, C. L., Gout, J. F., Johri, P., Doak, T. G., & Lynch, M. (2014). Differential retention and divergent resolution of duplicate genes following whole-genome duplication. Genome Research, 24(10), 1665–1675.
Melo-Ferreira, J., Vilela, J., Fonseca, M. M., da Fonseca, R. R., Boursot, P., & Alves, P. C. (2014). The elusive nature of adaptive mitochondrial DNA evolution of an arctic lineage prone to frequent introgression. Genome Biology and Evolution, 6(4), 886–896.
Mercy, J. A., & Saltzman, L. E. (1989). Fatal violence among spouses in the United States, 1976-85. American Journal of Public Health, 79(5), 595–599.
Mersha, T. B., & Abebe, T. (2015). Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Human Genomics, 9(1).
Messerschmidt, D. M., Knowles, B. B., & Solter, D. (2014). DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes & Development, 28(8), 812–828.
Miller, E. M. (1994). Paternal provisioning versus mate seeking in human populations. Personality and Individual Differences, 17(2), 227–255.
Mitteroecker, P., & Fischer, B. (2024). Evolution of the human birth canal. American Journal of Obstetrics and Gynecology, 230(3), S841–S855.
Morez Jacobs, A., Irish, J. D., Cooke, A., Anastasiadou, K., Barrington, C., Gilardet, A., ... & Girdland-Flink, L. (2025). Whole-genome ancestry of an Old Kingdom Egyptian. Nature, 1–8.
Morwood, M. J., O’Sullivan, P. B., Aziz, F., & Raza, A. (1998). Fission-track ages of stone tools and fossils on the east Indonesian island of Flores. Nature, 392, 173–176.
Mounier, A., Condemi, S., & Manzi, G. (2011). The stem species of our species: a place for the archaic human cranium from Ceprano, Italy. PLoS One, 6(4), e18821.
Moyà-Solà, S., Köhler, M., & Rook, L. (1999). Evidence of hominid-like precision grip capability in the hand of the Miocene ape Oreopithecus. Proceedings of the National Academy of Sciences, 96(1), 313–317.
Moyà-Solà, S., Köhler, M., & Rook, L. (2005). The Oreopithecus thumb: a strange case in hominoid evolution. Journal of Human Evolution, 49(3), 395–404.
Murray, C. (2003). Human accomplishment: The pursuit of excellence in the arts and sciences, 800 BC to 1950. New York: HarperCollins.
Murray, C. (2021). Facing Reality: Two Truths about Race in America. Encounter Books.
Nair, P. (2014). QnAs with Fred Gage. Proceedings of the National Academy of Sciences, 111(36), 12958–12959.
Navarrete, A., Van Schaik, C. P., & Isler, K. (2011). Energetics and the evolution of human brain size. Nature, 480, 91–93.
Neubauer, S., Hublin, J. J., & Gunz, P. (2018). The evolution of modern human brain shape. Science Advances, 4(1), eaao5961.
Ni, X., Ji, Q., Wu, W., Shao, Q., Ji, Y., Zhang, C., Liang, L., Ge, J., Guo, Z., Li, J., Li, Q., Grün, R., & Stringer, C. (2021). Massive cranium from Harbin in northeastern China establishes a new Middle Pleistocene human lineage. The Innovation, 2(3).
Niemitz, C. (2010). The evolution of the upright posture and gait—a review and a new synthesis. Naturwissenschaften, 97, 241–263.
Novembre, J., Johnson, T., Bryc, K., Kutalik, Z., Boyko, A. R., Auton, A., Indap, A., King, K. S., Bergmann, S., Nelson, M. R., Stephens, M., & Bustamante, C. D. (2008). Genes mirror geography within Europe. Nature, 456(7218), 98–101.
Nyland, Edo (2001). Linguistic Archaeology: An Introduction. Trafford Publishing.
O’Sullivan, B. P., & Freedman, S. D. (2009) Cystic fibrosis. The Lancet, 373(9678), P1891–1904.
O’Sullivan, P. B., Morwood, M., Hobbs, D., Suminto, F. A., Situmorang, M., Raza, A., & Maas, R. (2001). Archaeological implications of the geology and chronology of the Soa basin, Flores, Indonesia. Geology, 29(7), 607–610.
Olivieri, A., Achilli, A., Pala, M., Battaglia, V., Fornarino, S., Al-Zahery, N., ... & Torroni, A. (2006). The mtDNA legacy of the Levantine early Upper Palaeolithic in Africa. Science, 314(5806), 1767–1770.
Olson, M. V. (1999). When less is more: gene loss as an engine of evolutionary change. The American Journal of Human Genetics, 64(1), 18–23.
Orr, H. A. (1998). The population genetics of adaptation: the distribution of factors fixed during adaptive evolution. Evolution, 52(4), 935–949.
Otto, S. P., & Lenormand, T. (2002). Resolving the paradox of sex and recombination. Nature Reviews Genetics, 3, 252–261.
Pagani, L., Kivisild, T., Tarekegn, A., Ekong, R., Plaster, C., Romero, I. G., ... & Tyler-Smith, C. (2012). Ethiopian genetic diversity reveals linguistic stratification and complex influences on the Ethiopian gene pool. The American journal of Human Genetics, 91(1), 83–96.
Pagani, L., Schiffels, S., Gurdasani, D., Danecek, P., Scally, A., Chen, Y., ... & Tyler-Smith, C. (2015). Tracing the route of modern humans out of Africa by using 225 human genome sequences from Ethiopians and Egyptians. The American Journal of Human Genetics, 96(6), 986–991.
Parra, L., & Kirkegaard, E. O. W. (2025). National IQs: measurement and defense. OpenPsych. (under review)
Pasinelli, G. (2022). Genetic diversity and spatial genetic structure support the specialist-generalist variation hypothesis in two sympatric woodpecker species. Conservation Genetics, 23, 821–837.
Patterson, N., Richter, D. J., Gnerre, S., Lander, E. S., & Reich, D. (2006). Genetic evidence for complex speciation of humans and chimpanzees. Nature, 441, 1103–1108.
Peek, P. M., & Yankah, K. (2004). African folklore: An encyclopedia. New York: Routledge.
Pennarun, E., Kivisild, T., Metspalu, E., Metspalu, M., Reisberg, T., Moisan, J. P., ... & Villems, R. (2012). Divorcing the Late Upper Palaeolithic demographic histories of mtDNA haplogroups M1 and U6 in Africa. BMC Evolutionary Biology, 12, 234.
Pereira, V., Gomes, V., Amorim, A., Gusmão, L., & João Prata, M. (2010). Genetic characterization of uniparental lineages in populations from Southwest Iberia with past malaria endemicity. American Journal of Human Biology, 22(5), 588–595.
Pesta, B. J., Kirkegaard, E. O., te Nijenhuis, J., Lasker, J., & Fuerst, J. G. (2020). Racial and ethnic group differences in the heritability of intelligence: A systematic review and meta-analysis. Intelligence, 78, 101408.
Pickrell, J. K., Patterson, N., Loh, P. R., Lipson, M., Berger, B., Stoneking, M., ... & Reich, D. (2014). Ancient west Eurasian ancestry in southern and eastern Africa. Proceedings of the National Academy of Sciences, 111(7), 2632–2637.
Piel, F. B., Patil, A. P., Howes, R. E., Nyangiri, O. A., Gething, P. W., Williams, T. N., Weatherall, D. J., & Hay, S. I. (2010). Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nature Communications, 1, 104.
Pierron, D., Cortés, N. G., Letellier, T., & Grossman, L. I. (2013). Current relaxation of selection on the human genome: tolerance of deleterious mutations on olfactory receptors. Molecular Phylogenetics and Evolution, 66(2), 558–564.
Pierron, D., Heiske, M., Razafindrazaka, H., Rakoto, I., Rabetokotany, N., Ravololomanga, B., ... & Letellier, T. (2017). Genomic landscape of human diversity across Madagascar. Proceedings of the National Academy of Sciences, 114(32), E6498–E6506.
Piffer, D., & Kirkegaard, E. O. (2024). Polygenic selection and environmental influence on adult body height: Genetic and living standard contributions across diverse populations. Twin Research and Human Genetics, 27(6), 265–282.
Plavcan, J. M. (2018). Sexual dimorphism in hominin ancestors. In H., Callan (Ed.), The international encyclopedia of anthropology (pp. 1–6). Wiley.
Pollet, T. V., Nelissen, M., & Nettle, D. (2009). Lineage based differences in grandparental investment: evidence from a large British cohort study. Journal of Biosocial Science, 41(3), 355–379.
Pollet, T. V., Nettle, D., & Nelissen, M. (2007). Maternal grandmothers do go the extra mile: Factoring distance and lineage into differential contact with grandchildren. Evolutionary Psychology, 5(4), 832–843.
Poloni, E. S., Semino, O., Passarino, G., Santachiara-Benerecetti, A. S., Dupanloup, I., Langaney, A., & Excoffier, L. (1997). Human genetic affinities for Y-chromosome P49a, f/TaqI haplotypes show strong correspondence with linguistics. The American Journal of Human Genetics, 61(5), 1015–1035.
Posth, C., Renaud, G., Mittnik, A., Drucker, D. G., Rougier, H., Cupillard, C., ... & Krause, J. (2016). Pleistocene mitochondrial genomes suggest a single major dispersal of non-Africans and a Late Glacial population turnover in Europe. Current Biology, 26(6), 827–833.
Posth, C., Wißing, C., Kitagawa, K., Pagani, L., Van Holstein, L., Racimo, F., Wehrberger, K., Conard, N. J., Kind, C. J., Bocherens, H., & Krause, J. (2017). Deeply divergent archaic mitochondrial genome provides lower time boundary for African gene flow into Neanderthals. Nature Communications, 8, 16046.
Poznik, G. D., Xue, Y., Mendez, F. L., Willems, T. F., Massaia, A., Wilson Sayres, M. A., ... & Tyler-Smith, C. (2016). Punctuated bursts in human male demography inferred from 1,244 worldwide Y-chromosome sequences. Nature Genetics, 48, 593–599.
Pratt, T. C., Turanovic, J. J., & Cullen, F. T. (2016). Revisiting the criminological consequences of exposure to fetal testosterone: A meta‐analysis of the 2D: 4D digit ratio. Criminology, 54(4), 587–620.
Prendergast, M. E., Rouby, H., Punnwong, P., Marchant, R., Crowther, A., Kourampas, N., ... & Boivin, N. L. (2016). Continental island formation and the archaeology of defaunation on Zanzibar, eastern Africa. PLoS One, 11(2), e0149565.
Pritchard, J. K., Pickrell, J. K., & Coop, G. (2010). The genetics of human adaptation: hard sweeps, soft sweeps, and polygenic adaptation. Current Biology, 20(4), R208–R215.
Racimo, F., Marnetto, D., & Huerta-Sánchez, E. (2017). Signatures of archaic adaptive introgression in present-day human populations. Molecular Biology and Evolution, 34(2), 296–317.
Racimo, F., Sankararaman, S., Nielsen, R., & Huerta-Sánchez, E. (2015). Evidence for archaic adaptive introgression in humans. Nature Reviews Genetics, 16(6), 359–371.
Ragsdale, A. P., Weaver, T. D., Atkinson, E. G., Hoal, E. G., Möller, M., Henn, B. M., & Gravel, S. (2023). A weakly structured stem for human origins in Africa. Nature, 617, 755–763.
Rajkumar, R., Banerjee, J., Gunturi, H. B., Trivedi, R., & Kashyap, V. K. (2005). Phylogeny and antiquity of M macrohaplogroup inferred from complete mtDNA sequence of Indian specific lineages. BMC Evolutionary Biology, 5, 26.
Rebbeck, T. R. (2018). Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harbor Perspectives in Medicine, 8(9), a030387.
Reilly, P. F., Tjahjadi, A., Miller, S. L., Akey, J. M., & Tucci, S. (2022). The contribution of Neanderthal introgression to modern human traits. Current Biology, 32(18), R970–R983.
Relethford, J. H. (2008). Genetic evidence and the modern human origins debate. Heredity, 100, 555–563.
Richards, G., Aydin, E., Tsompanidis, A., Padaigaitė, E., Austin, T., Allison, C., Holt, R., & Baron-Cohen, S. (2022). Digit ratio (2D: 4D) and maternal testosterone-to-estradiol ratio measured in early pregnancy. Scientific Reports, 12, 13586.
Rieux, A., Eriksson, A., Li, M., Sobkowiak, B., Weinert, L. A., Warmuth, V., Ruiz-Linares, A., Manica, A., & Balloux, F. (2014). Improved calibration of the human mitochondrial clock using ancient genomes. Molecular Biology and Evolution, 31(10), 2780–2792.
Rindermann, H. (2007). The g-factor of international cognitive ability comparisons: The homogeneity of results in PISA, TIMSS, PIRLS and IQ tests across nations. European Journal of Personality, 21, 667–706.
Robinson, J., Kyriazis, C. C., Yuan, S. C., & Lohmueller, K. E. (2023). Deleterious variation in natural populations and implications for conservation genetics. Annual Review of Animal Biosciences, 11, 93–114.
Roca-Ayats, N., Maceda, I., Bruque, C. D., Martínez-Gil, N., Garcia-Giralt, N., Cozar, M., ... & Balcells, S. (2024). Evolutionary and functional analyses of LRP5 in archaic and extant modern humans. Human Genomics, 18, 53.
Roca-Rada, X., Davidson, R., Williams, M. P., Ravishankar, S., Collen, E., Haarkötter, C., ... & Teixeira, J. C. (2024). The genetic history of Portugal over the past 5,000 years. bioRxiv.
Rodrı́guez-Gironés, M. A., & Enquist, M. (2001). The evolution of female sexuality. Animal Behaviour, 61(4), 695–704.
Romiguier, J., Gayral, P., Ballenghien, M., Bernard, A., Cahais, V., Chenuil, A., ... & Galtier, N. (2014). Comparative population genomics in animals uncovers the determinants of genetic diversity. Nature, 515, 261–263.
Rosas, A., Martínez-Maza, C., Bastir, M., García-Tabernero, A., Lalueza-Fox, C., Huguet, R., ... & Fortea, J. (2006). Paleobiology and comparative morphology of a late Neandertal sample from El Sidrón, Asturias, Spain. Proceedings of the National Academy of Sciences, 103(51), 19266–19271.
Rushton, J. P. (1989). Genetic similarity, human altruism, and group selection. Behavioral and Brain Sciences, 12(3), 503–559.
Rushton, J. P. (1997). Race, evolution and behavior: A life history perspective. New Brunswick, NJ: Transaction.
Rushton, J. P. (2005). Ethnic nepotism, evolutionary psychology, and genetic similarity theory. Nations and Nationalism, 11(4), 489–507.
Rushton, J. P. (2009). Inclusive fitness in human relationships. Biological Journal of the Linnean Society, 96, 8–12.
Rushton, J. P., & Bogaert, A. F. (1987). Race differences in sexual behavior: Testing an evolutionary hypothesis. Journal of Research in Personality, 21(4), 529–551.
Rushton, J. P., & Bons, T. A. (2005). Mate choice and friendship in twins: evidence for genetic similarity. Psychological Science, 16(7), 555–559.
Rushton, J. P., & Jensen, A. R. (2010). Race and IQ: A theory-based review of the research in Richard Nisbett’s intelligence and how to get it. The Open Psychology Journal, 3, 9–35.
Rushton, J. P., & Rushton, E. W. (2003). Brain size, IQ, and racial-group differences: Evidence from musculoskeletal traits. Intelligence, 31(2), 139–155.
Rushton, J. P., & Russell, J. H. (1985). Genetic similarity theory: A reply to Mealey and new evidence. Behavior Genetics, 15, 575–582.
Rushton, J. P., Vernon, P. A., & Bons, T. A. (2007). No evidence that polymorphisms of brain regulator genes Microcephalin and ASPM are associated with general mental ability, head circumference or altruism. Biology Letters, 3(2), 157–160.
Ryan, T. M., & Shaw, C. N. (2015). Gracility of the modern Homo sapiens skeleton is the result of decreased biomechanical loading. Proceedings of the National Academy of Sciences, 112(2), 372–377.
Saladié, P., & Rodríguez-Hidalgo, A. (2017). Archaeological evidence for cannibalism in prehistoric Western Europe: from Homo antecessor to the Bronze Age. Journal of Archaeological Method and Theory, 24, 1034–1071.
Salter, F. (2007). On genetic interests: Family, ethnicity, and humanity in an age of mass migration. Transaction Publishers.
Sankararaman, S., Mallick, S., Dannemann, M., Prüfer, K., Kelso, J., Pääbo, S., Patterson, N., & Reich, D. (2014). The genomic landscape of Neanderthal ancestry in present-day humans. Nature, 507, 354–357.
Sankararaman, S., Mallick, S., Patterson, N., & Reich, D. (2016). The combined landscape of Denisovan and Neanderthal ancestry in present-day humans. Current Biology, 26(9), 1241–1247.
Sarich, V., & Miele, F. (2004). Race, the reality of human differences. Boulder: Westview Press.
Scerri, E. M., Thomas, M. G., Manica, A., Gunz, P., Stock, J. T., Stringer, C., ... & Chikhi, L. (2018). Did our species evolve in subdivided populations across Africa, and why does it matter?. Trends in Ecology & Evolution, 33(8), P582–594.
Scheinfeldt, L. B., Soi, S., Lambert, C., Ko, W. Y., Coulibaly, A., Ranciaro, A., ... & Tishkoff, S. A. (2019). Genomic evidence for shared common ancestry of East African hunting-gathering populations and insights into local adaptation. Proceedings of the National Academy of Sciences, 116(10), 4166–4175.
Schillaci, M. A. (2006). Sexual selection and the evolution of brain size in primates. PLoS One, 1(1), e62.
Schlebusch, C. M., Malmström, H., Günther, T., Sjödin, P., Coutinho, A., Edlund, H., ... & Jakobsson, M. (2017). Southern African ancient genomes estimate modern human divergence to 350,000 to 260,000 years ago. Science, 358(6363), 652–655.
Schlebusch, C. M., Skoglund, P., Sjödin, P., Gattepaille, L. M., Hernandez, D., Jay, F., ... & Jakobsson, M. (2012). Genomic variation in seven Khoe-San groups reveals adaptation and complex African history. Science, 338(6105), 374–379.
Schnitzer, P. G., & Ewigman, B. G. (2005). Child deaths resulting from inflicted injuries: household risk factors and perpetrator characteristics. Pediatrics, 116(5), e687–e693.
Schuenemann, V. J., Peltzer, A., Welte, B., Van Pelt, W. P., Molak, M., Wang, C. C., ... & Krause, J. (2017). Ancient Egyptian mummy genomes suggest an increase of Sub-Saharan African ancestry in post-Roman periods. Nature Communications, 8, 15694.
Schwartz, J. H. (1999). Sudden Origins: Fossils, Genes, and the Emergence of Species. New York: John Wiley & Sons.
Schwartz, J. H. (2005). The red ape: Orangutans and human origins. Westview Press.
Sear, R., & Mace, R. (2008). Who keeps children alive? A review of the effects of kin on child survival. Evolution and Human Behavior, 29(1), 1–18.
Segal, N. L. 1999. Entwined Lives: Twins and What They Tell Us About Human Behavior. New York: Dutton.
Segal, N. L., Sussman, L. J., Marelich, W. D., Mearns, J., & Blozis, S. A. (2002). Monozygotic and dizygotic twins’ retrospective and current bereavement-related behaviors: An evolutionary perspective. Twin Research and Human Genetics, 5(3), 188–195.
Sekajova, Z., Fossen, E. I., Rosa, E., Ratikainen, I. I., Tourniaire-Blum, M., Bolund, E., & Lind, M. I. (2023). Evolution of phenotypic plasticity during environmental fluctuations. bioRxiv.
Sesardic, N. (2010). Race: a social destruction of a biological concept. Biology & Philosophy, 25, 143–162.
Sevim-Erol, A., Begun, D. R., Yavuz, A., Tarhan, E., Sözer, Ç. S., Mayda, S., van den Hoek Ostende, L. W., Martin, R. M. G., & Alçiçek, M. C. (2023). A new ape from Türkiye and the radiation of late Miocene hominines. Communications Biology, 6(1), 842.
Shultz, S., & Dunbar, R. I. (2007). The evolution of the social brain: anthropoid primates contrast with other vertebrates. Proceedings of the Royal Society B: Biological Sciences, 274, 2429–2436.
Sikora, M., Pitulko, V. V., Sousa, V. C., Allentoft, M. E., Vinner, L., Rasmussen, S., ... & Willerslev, E. (2019). The population history of northeastern Siberia since the Pleistocene. Nature, 570(7760), 182–188.
Skinner, M. K. (2014). Environmental stress and epigenetic transgenerational inheritance. BMC Medicine, 12(153), 1–5.
Skinner, M. K. (2015). Environmental epigenetics and a unified theory of the molecular aspects of evolution: a neo-Lamarckian concept that facilitates neo-Darwinian evolution. Genome Biology and Evolution, 7(5), 1296–1302.
Skoglund, P., Thompson, J. C., Prendergast, M. E., Mittnik, A., Sirak, K., Hajdinjak, M., ... & Reich, D. (2017). Reconstructing prehistoric African population structure. Cell, 171(1), 59–71.
So, J. K. (1980). Human biological adaptation to arctic and subarctic zones. Annual Review of Anthropology, 9, 63–82.
Soares, P., Alshamali, F., Pereira, J. B., Fernandes, V., Silva, N. M., Afonso, C., ... & Pereira, L. (2012). The expansion of mtDNA haplogroup L3 within and out of Africa. Molecular Biology and Evolution, 29(3), 915–927.
Solovieff, N., Hartley, S. W., Baldwin, C. T., Klings, E. S., Gladwin, M. T., Taylor VI, J. G., Kato, G. J., Farrer, L. A., Steinberg, M. H., & Sebastiani, P. (2011). Ancestry of African Americans with sickle cell disease. Blood Cells, Molecules, and Diseases, 47(1), 41–45.
Stapley, J., Feulner, P. G., Johnston, S. E., Santure, A. W., & Smadja, C. M. (2017). Recombination: the good, the bad and the variable. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1736), 20170279.
Stefansson, H., Helgason, A., Thorleifsson, G., Steinthorsdottir, V., Masson, G., Barnard, J., ... & Stefansson, K. (2005). A common inversion under selection in Europeans. Nature Genetics, 37, 129–137.
Stock, F., & Gifford-Gonzalez, D. (2013). Genetics and African cattle domestication. African Archaeological Review, 30, 51–72.
Stringer, C. (2012). The status of Homo heidelbergensis (Schoetensack 1908). Evolutionary Anthropology: Issues, News, and Reviews, 21(3), 101–107.
Sutikna, T., Tocheri, M. W., Morwood, M. J., Saptomo, E. W., Jatmiko, Awe, R. D., ... & Roberts, R. G. (2016). Revised stratigraphy and chronology for Homo floresiensis at Liang Bua in Indonesia. Nature, 532, 366–369.
Svanbäck, R., & Schluter, D. (2012). Niche specialization influences adaptive phenotypic plasticity in the threespine stickleback. The American Naturalist, 180(1), 50–59.
Tang, H., Quertermous, T., Rodriguez, B., Kardia, S. L., Zhu, X., Brown, A., ... & Risch, N. J. (2005). Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. The American Journal of Human Genetics, 76(2), 268–275.
Temple, R., & Stockbridge, N. L. (2007). BiDil for heart failure in black patients: The US Food and Drug Administration perspective. Annals of Internal Medicine, 146(1), 57–62.
Templer, D. I., & Arikawa, H. (2006). Temperature, skin color, per capita income, and IQ: An international perspective. Intelligence, 34(2), 121–139.
Templer, D. I., & Stephens, J. S. (2014). The relationship between IQ and climatic variables in African and Eurasian countries. Intelligence, 46, 169-178.
Temrin, H., Buchmayer, S., & Enquist, M. (2000). Step–parents and infanticide: new data contradict evolutionary predictions. Proceedings of the Royal Society of London. Series B: Biological Sciences, 267(1446), 943–945.
Thomas, J. A., Welch, J. J., Lanfear, R., & Bromham, L. (2010). A generation time effect on the rate of molecular evolution in invertebrates. Molecular Biology and Evolution, 27(5), 1173–1180.
Thompson, P. M., Cannon, T. D., Narr, K. L., Van Erp, T., Poutanen, V. P., Huttunen, M., ... & Toga, A. W. (2001). Genetic influences on brain structure. Nature Neuroscience, 4, 1253–1258.
Thorpe, S. K., Holder, R. L., & Crompton, R. H. (2007). Origin of human bipedalism as an adaptation for locomotion on flexible branches. Science, 316(5829), 1328–1331.
Timmermann, A., Yun, K. S., Raia, P., Ruan, J., Mondanaro, A., Zeller, E., ... & Ganopolski, A. (2022). Climate effects on archaic human habitats and species successions. Nature, 604(7906), 495–501.
Tishkoff, S. A., Reed, F. A., Friedlaender, F. R., Ehret, C., Ranciaro, A., Froment, A., ... & Williams, S. M. (2009). The genetic structure and history of Africans and African Americans. Science, 324(5930), 1035–1044.
Tishkoff, S. A., Reed, F. A., Ranciaro, A., Voight, B. F., Babbitt, C. C., Silverman, J. S., ... & Deloukas, P. (2007). Convergent adaptation of human lactase persistence in Africa and Europe. Nature Genetics, 39(1), 31–40.
Tobler, R., Rohrlach, A., Soubrier, J., Bover, P., Llamas, B., Tuke, J., ... & Cooper, A. (2017). Aboriginal mitogenomes reveal 50,000 years of regionalism in Australia. Nature, 544, 180–184.
Toews, D. P., & Brelsford, A. (2012). The biogeography of mitochondrial and nuclear discordance in animals. Molecular Ecology, 21(16), 3907–3930.
Tojima, S. (2021). A tale of the tail: a comprehensive understanding of the “human tail”. Journal of Korean Neurosurgical Society, 64(3), 340–345.
Tomasello, M., Hare, B., Lehmann, H., & Call, J. (2007). Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. Journal of Human Evolution, 52(3), 314–320.
Tomkins, J. P., & Bergman, J. (2015). Evolutionary molecular genetic clocks—a perpetual exercise in futility and failure. Journal of Creation, 29(2), 26–35.
Tooley, G. A., Karakis, M., Stokes, M., & Ozanne-Smith, J. (2006). Generalising the Cinderella Effect to unintentional childhood fatalities. Evolution and Human Behavior, 27(3), 224–230.
Toomey, R., Panizzon, M. S., Kremen, W. S., Franz, C. E., & Lyons, M. J. (2015). A twin-study of genetic contributions to morningness–eveningness and depression. Chronobiology International, 32(3), 303–309.
Trivers, R., Jacobson, A., & Manning, J. T. (2020). Radiographic digit ratios (2D: 4D) of Afro-Caribbean children: Comparisons with published data from white children. Early Human Development, 146, 105072.
Tu, M., Zhang, H., Guo, Y., Zhang, L., Wei, X., & Yu, Q. (2023). Which grandparent is more intimate? The effects of the gender of grandchildren. Current Psychology, 42, 5337–5346.
Tufto, J. (2015). Genetic evolution, plasticity, and bet-hedging as adaptive responses to temporally autocorrelated fluctuating selection: A quantitative genetic model. Evolution, 69(8), 2034–2049.
Tylén, K., Fusaroli, R., Rojo, S., Heimann, K., Fay, N., Johannsen, N. N., Riede, F., & Lombard, M. (2020). The evolution of early symbolic behavior in Homo sapiens. Proceedings of the National Academy of Sciences, 117(9), 4578–4584.
Trense, D., Habel, J. C., Kramp, K., Schmitt, T., & Fischer, K. (2021). Does specialisation affect genetic diversity in (pre-) Alpine populations of four species of Copper butterflies?. Journal of Insect Conservation, 25, 321–338.
Underhill, P. A., Passarino, G., Lin, A. A., Shen, P., Lahr, M. M., Foley, R. A., ... & Cavalli-Sforza, L. L. (2001). The phylogeography of Y chromosome binary haplotypes and the origins of modern human populations. Annals of Human Genetics, 65(1), 43–62.
Van Schaik, C. P., Ancrenaz, M., Borgen, G., Galdikas, B., Knott, C. D., Singleton, I., ... & Merrill, M. (2003). Orangutan cultures and the evolution of material culture. Science, 299(5603), 102–105.
van Schaik, C. P., Ancrenaz, M., Djojoasmoro, R., Knott, C. D., Morrogh-Bernard, H. C., Nuzuar, O. K., ... & Van Noordwijk, M. A. (2009). Orangutan cultures revisited. In S.A., Wich, S. S. U., Atmoko, T. M., Setia, & C. P., van Schaik (Eds.), Orangutans: Geographic Variation in Behavioral Ecology and Conservation (pp. 299–309). Oxford University Press.
Vaneechoutte, M., Kuliukas, A., & Verhaegen, M. (2012a). Was Man More Aquatic in the Past? Fifty Years After Alister Hardy: Waterside Hypotheses of Human Evolution. Bentham Science Publishers.
Vaneechoutte, M., Munro, S., & Verhaegen, M. (2012b). Reply to John Langdon’s review of the eBook: Was Man more aquatic in the past? Fifty years after Alister Hardy, Waterside Hypotheses of Human Evolution, Mario Vaneechoutte, Algis Kuliukas, Marc Verhaegen (Eds.), Bentham eBooks (2011), eISBN: 9781608052448.
Vernon, P. A., Martin, R. A., Schermer, J. A., Cherkas, L. F., & Spector, T. D. (2008). Genetic and environmental contributions to humor styles: A replication study. Twin Research and Human Genetics, 11(1), 44–47.
Vernot, B., & Akey, J. M. (2014). Resurrecting surviving Neandertal lineages from modern human genomes. Science, 343(6174), 1017–1021.
Vernot, B., & Akey, J. M. (2015). Complex history of admixture between modern humans and Neandertals. The American Journal of Human Genetics, 96(3), 448–453.
Voracek, M. (2014). No effects of androgen receptor gene CAG and GGC repeat polymorphisms on digit ratio (2D: 4D): a comprehensive meta-analysis and critical evaluation of research. Evolution and Human Behavior, 35(5), 430–437.
Wai, J. (2013). Investigating America’s elite: Cognitive ability, education, and sex differences. Intelligence, 41(4), 203–211.
Wai, J. (2014). Experts are born, then made: Combining prospective and retrospective longitudinal data shows that cognitive ability matters. Intelligence, 45, 74–80.
Wall, J. D., & Brandt, D. Y. C. (2016). Archaic admixture in human history. Current Opinion in Genetics & Development, 41, 93–97.
Wagner, D. R., & Heyward, V. H. (2000). Measures of body composition in blacks and whites: a comparative review. The American Journal of Clinical Nutrition, 71(6), 1392–1402.
Weekes-Shackelford, V. A., & Shackelford, T. K. (2004). Methods of filicide: Stepparents and genetic parents kill differently. Violence and Victims, 19(1), 75–81.
Wei, Y., de Lange, S. C., Scholtens, L. H., Watanabe, K., Ardesch, D. J., Jansen, P. R., ... & van den Heuvel, M. P. (2019). Genetic mapping and evolutionary analysis of human-expanded cognitive networks. Nature Communications, 10(1), 4839.
Wells, J. C. (2016). The metabolic ghetto: an evolutionary perspective on nutrition, power relations and chronic disease. Cambridge: Cambridge University Press.
Whitrow, M. J., & Harding, S. (2008). Ethnic differences in adolescent lung function: anthropometric, socioeconomic, and psychosocial factors. American Journal of Respiratory and Critical Care Medicine, 177(11), 1262–1267.
Wilkins, J., Schoville, B. J., Brown, K. S., & Chazan, M. (2012). Evidence for early hafted hunting technology. Science, 338, 942–946.
Williams, S. A. (2010). Morphological integration and the evolution of knuckle-walking. Journal of Human Evolution, 58(5), 432–440.
Witherspoon, D. J., Wooding, S., Rogers, A. R., Marchani, E. E., Watkins, W. S., Batzer, M. A., & Jorde, L. B. (2007). Genetic similarities within and between human populations. Genetics, 176(1), 351–359.
Wohlers, I., Künstner, A., Munz, M., Olbrich, M., Fähnrich, A., Calonga-Solís, V., ... & Ibrahim, S. (2020). An integrated personal and population-based Egyptian genome reference. Nature Communications, 11, 4719.
Woodley, M. A., Rindermann, H., Bell, E., Stratford, J., & Piffer, D. (2014). The relationship between Microcephalin, ASPM and intelligence: A reconsideration. Intelligence, 44, 51–63.
Wray, G. A. (2007). The evolutionary significance of cis-regulatory mutations. Nature Reviews Genetics, 8(3), 206–216.
Wu, X., Pei, S., Cai, Y., Tong, H., Zhang, Z., Yan, Y., Xing, S., Martinón-Torres, M., Bermúdez de Castro, J. M., & Liu, W. (2023). Morphological and morphometric analyses of a late Middle Pleistocene hominin mandible from Hualongdong, China. Journal of Human Evolution, 182, 103411.
Xia, Z., Chen, H., Zhang, Y., & Huang, S. (2021). Ancient uniparental DNAs in distinguishing the competing theories of molecular evolution and modern human origins. Research Square.
Xia, B., Zhang, W., Zhao, G., Zhang, X., Bai, J., Brosh, R., ... & Yanai, I. (2024). On the genetic basis of tail-loss evolution in humans and apes. Nature, 626, 1042–1048.
Xu, Y., & Zheng, Y. (2015). The digit ratio (2D: 4D) in China: A meta‐analysis. American Journal of Human Biology, 27(3), 304–309.
Zaidi, A. A., Mattern, B. C., Claes, P., McEcoy, B., Hughes, C., & Shriver, M. D. (2017). Investigating the case of human nose shape and climate adaptation. PLoS Genetics, 13(3), e1006616.
Zhang, Y., & Huang, S. (2019). The Out of East Asia model versus the African Eve model of modern human origins in light of ancient mtDNA findings. bioRxiv.
Zhao, B., Ibrahim, J. G., Li, Y., Li, T., Wang, Y., Shan, Y., ... & Zhu, H. (2019). Heritability of regional brain volumes in large-scale neuroimaging and genetic studies. Cerebral Cortex, 29(7), 2904–2914.
Zietsch, B. P., Verweij, K. J., Heath, A. C., & Martin, N. G. (2011). Variation in human mate choice: Simultaneously investigating heritability, parental influence, sexual imprinting, and assortative mating. The American Naturalist, 177(5), 605–616.
Zink, R. M., & Barrowclough, G. (2008) Mitochondrial DNA under siege in avian phylogeography. Molecular Ecology, 17(9), 2107–2121.
Zink, K. D., & Lieberman, D. E. (2016). Impact of meat and Lower Palaeolithic food processing techniques on chewing in humans. Nature, 531, 500–503.





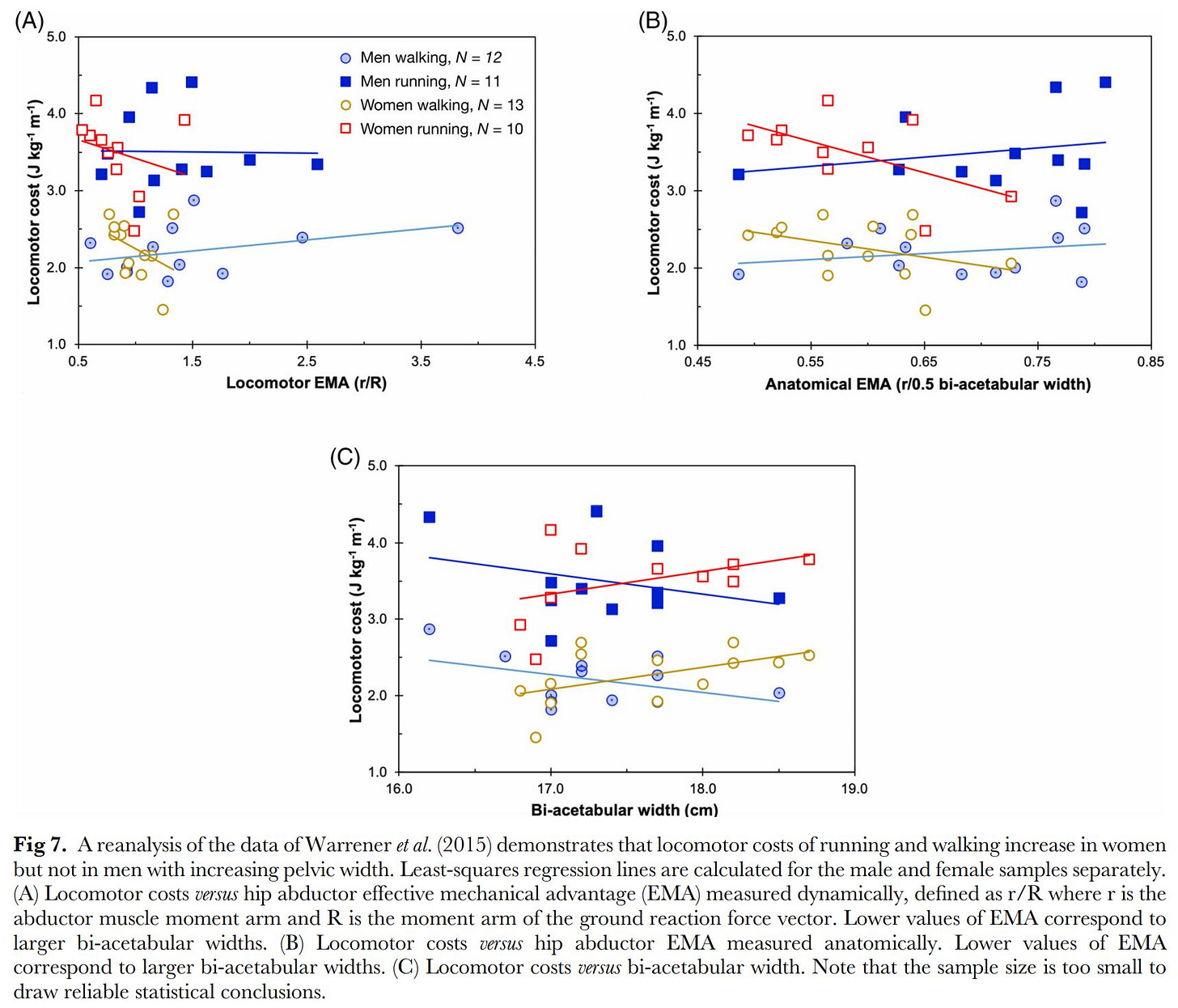





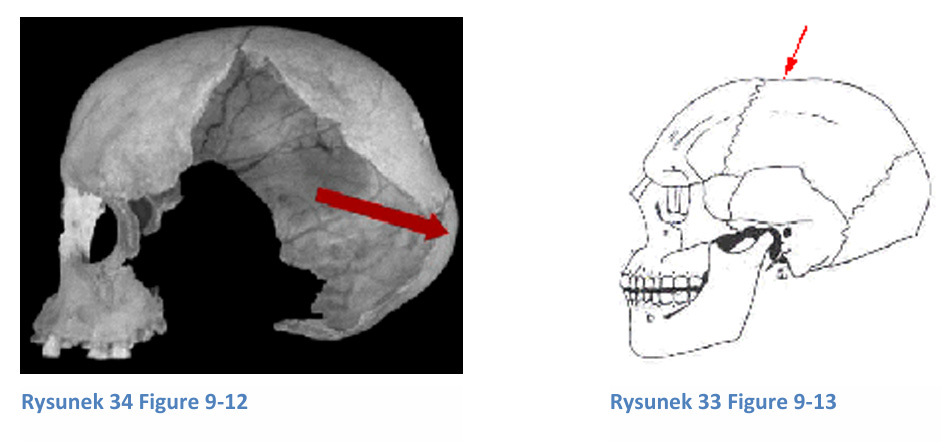

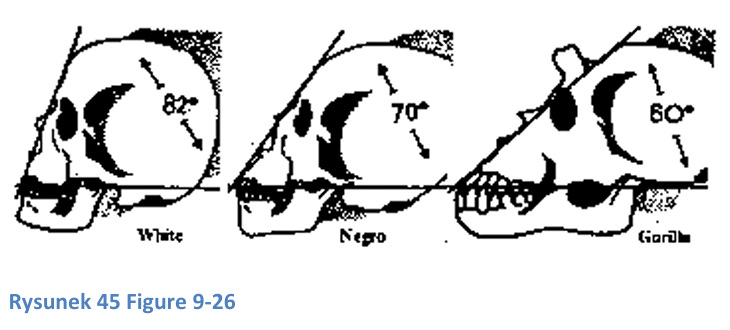






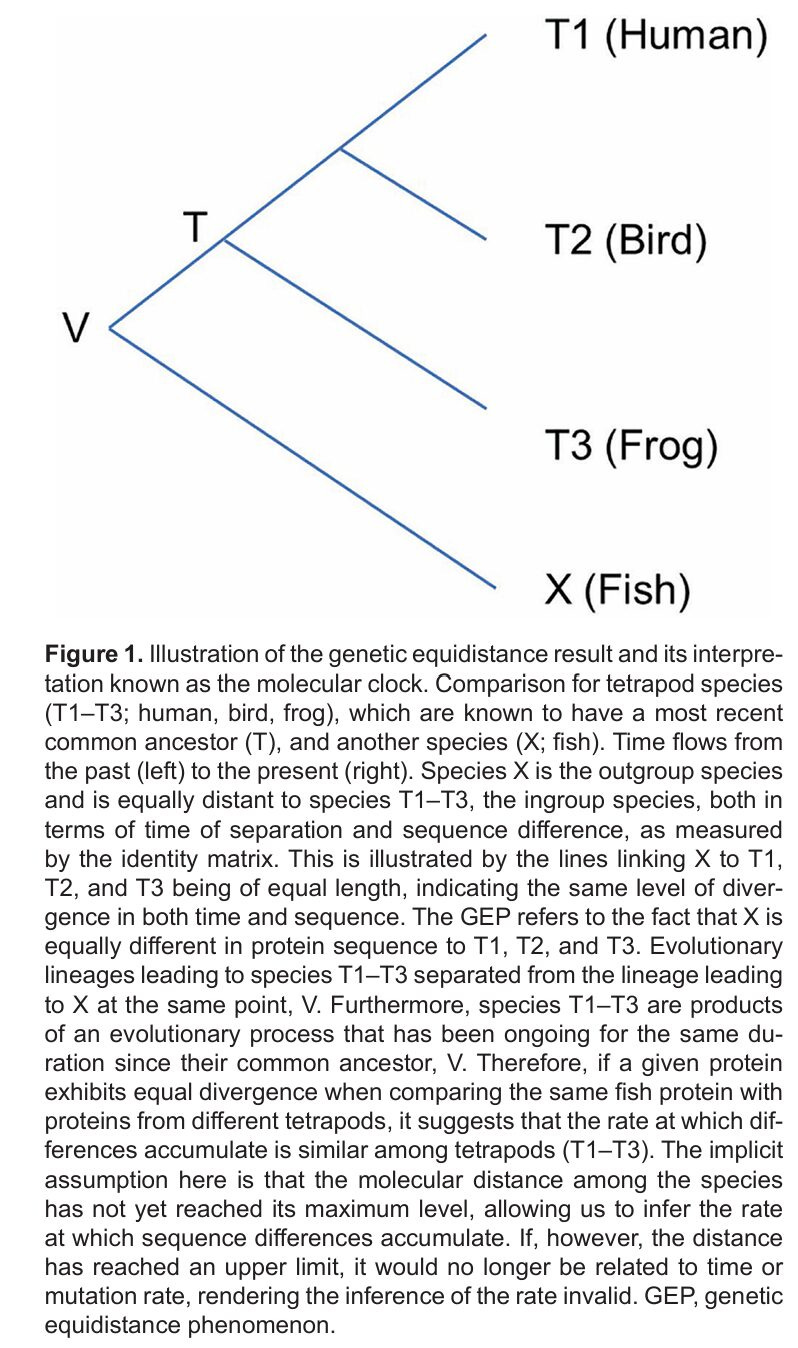

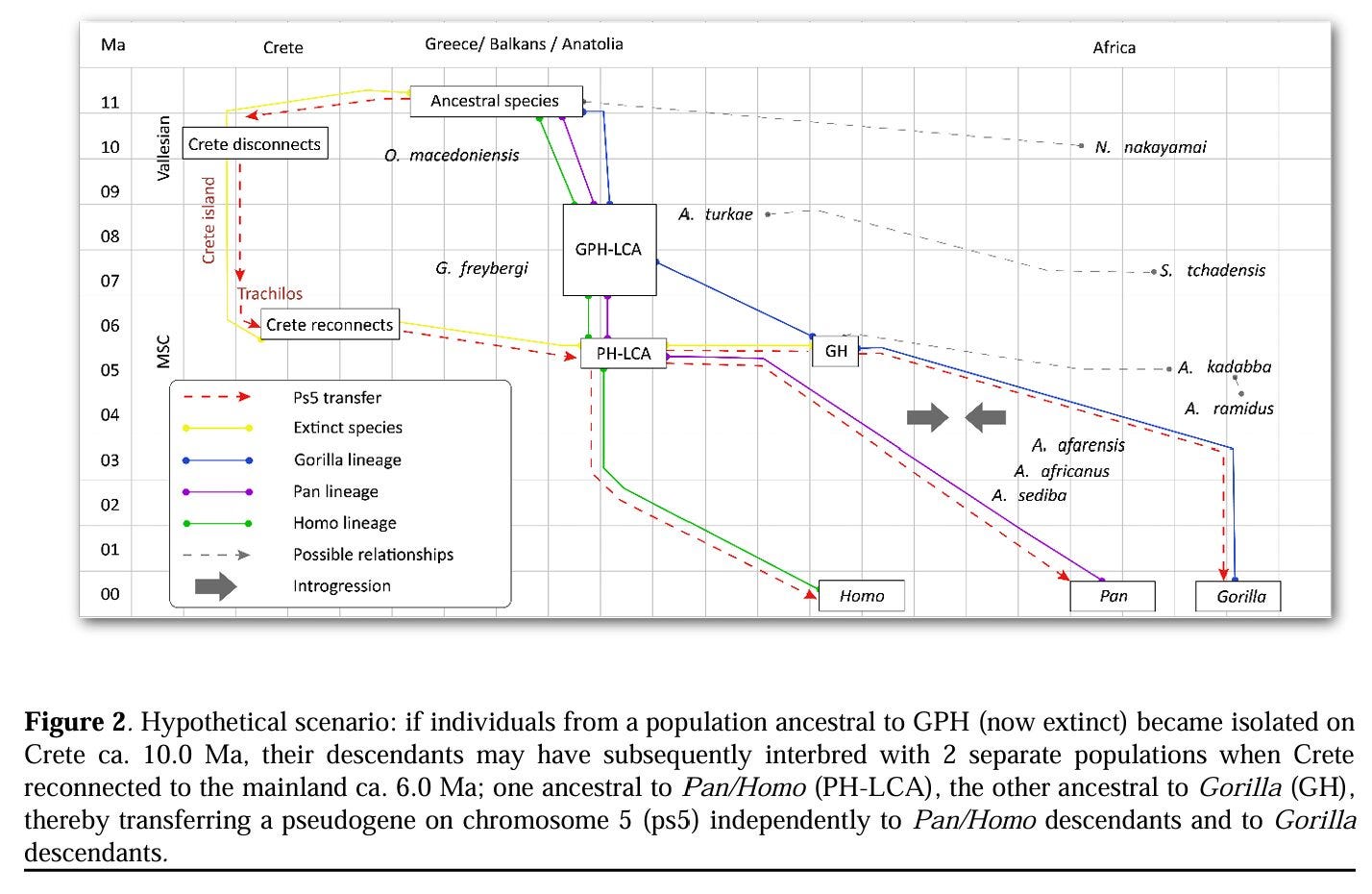




Playtime is over everyone. I'm tired of watching the thread becoming a trollfest. If you can't act civilized, you will be banned. I already banned 3 persons. If you have nothing to contribute, avoid leaving a comment. I will delete anything that fuels trolls or hatred. The thread is filled with useless comments as of now.
They obviously are. Genetic testing and observation confirms it.